DEPARTMENT OF HEALTH AND HUMAN SERVICES
Centers for Disease Control and Prevention
National Institute for Occupational Safety and Health
NIOSH Manual of Analytical Methods (NMAM), 5th Edition
Sampling and characterization of
bioaerosols
by William G. Lindsley, Brett J. Green, Francoise M. Blachere, Stephen B. Martin, Brandon F.
Law, Paul A. Jensen and Millie P. Schafer, NIOSH
BA-2
BA-5
BA-10
BA-28
BA-36
BA-39
BA-42
BA-46
BA-49
BA-62
BA-65
BA-65
BA-66
BA-101
1 Introduction
2 Principles of bioaerosol collection
3 D
evices used for bioaerosol sampling
4 Considerations for bioaerosol sampling
5 Selection of bioaerosol samplers
6 Sample preparation for culturable bioaerosols
7 Identification of culturable bioaerosols
8 Enumeration of culturable bioaerosols
9 Sample analysis methods for non-viable and non-culturable bioaerosols
10 Limitations of bioaerosol sampling and characterization
11 Safety considerations
12 Resources
13 References
14 Appendix 1- List of manufacturers/distributors of common bioaerosol
samplers and related products
15 Appendix 2 – Commonly used bioaerosol samplers
BA-104

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-2 of BA-115
Sampling and Characterization of Bioaerosols
1 Introduction
Bioaerosols are airborne particles that originate from biological sources including animals,
plants, fungi, bacteria, protozoa, and viruses. Examples of bioaerosols encountered in
occupational environments include plant pollen, algae, fungal spores, bacteria such as
actinomycetes, droplets produced during coughing and sneezing that may contain bacteria
and viruses, dust containing insect excreta, animal dander, and fragments derived from each
of these sources. Bioaerosols are ubiquitous and can be isolated from indoor, outdoor, and
occupational environments using a variety of methods that either enumerate viable or a
collection of viable and non-viable bioaerosols. Photomicrographs of example viral, bacterial,
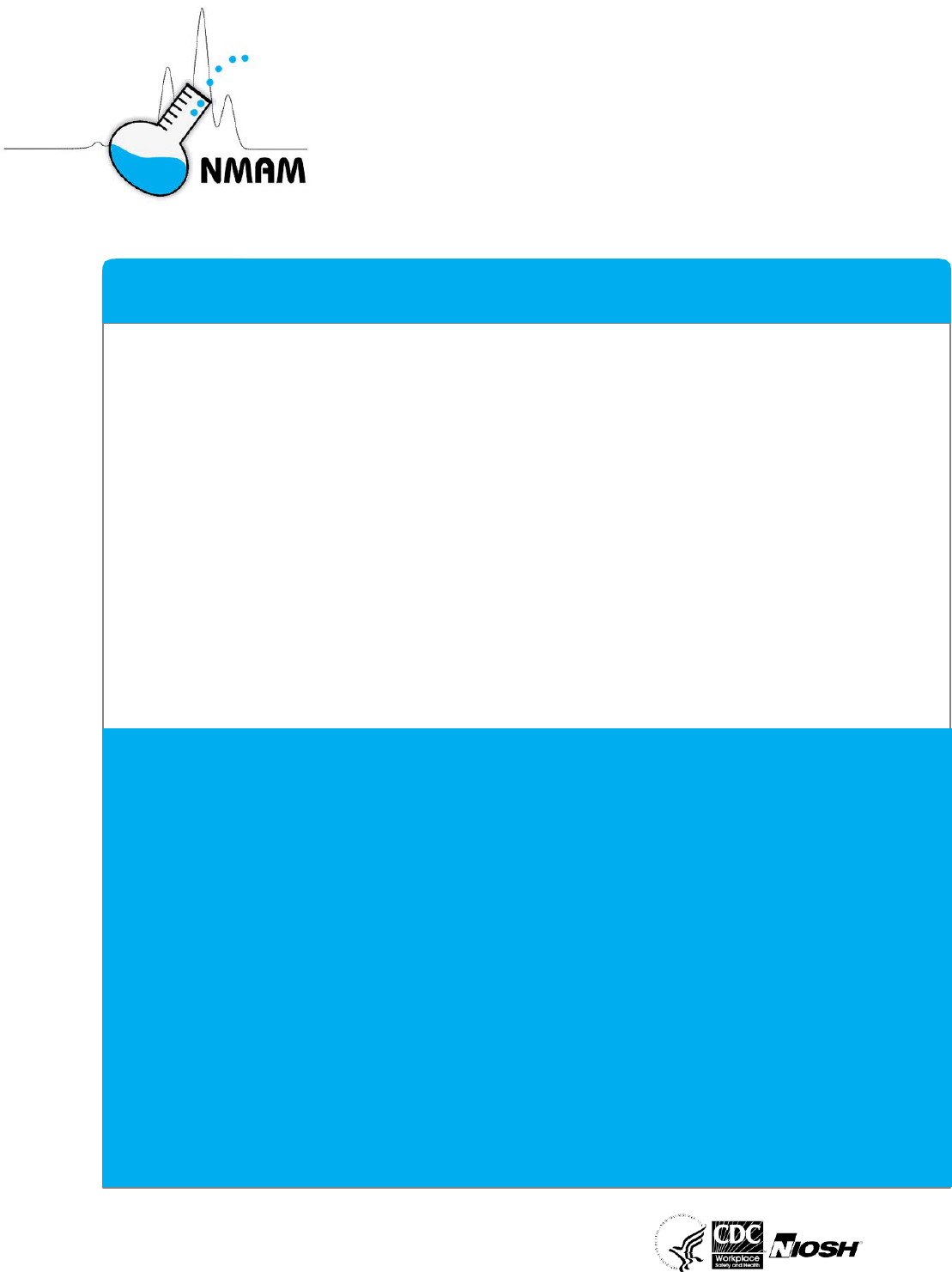
fungal, and plant bioaerosols are presented in Figure 1.
Bioaerosol monitoring is a rapidly emerging area of industrial hygiene due to the improved
analysis methods such as polymerase chain reaction (PCR) and the impact that occupational
exposures may have on worker respiratory health, particularly in microbial contaminated
environments [Eduard et al. 2012; Environment Agency 2009; Haig et al. 2016; Hung et al.
2005; Macher 1999; Morey 2007; Nazaroff 2016]. Some human diseases encountered in
healthcare settings such as measles and tuberculosis can be spread by bioaerosols containing
infectious microorganisms [Ijaz et al. 2016; Jones and Brosseau 2015]. Soil saprophytic fungi
such as Coccidioides immitis can be aerosolized during occupational disturbance activities
and, if inhaled, can result in an acute pulmonary infection [Das et al. 2012; Wilken et al. 2014;
Wilken et al. 2015]. The measurement of these bioaerosols in industrial hygiene includes the
measurement of viable (culturable and non-culturable) and nonviable bioaerosols in indoor
settings (e.g., industrial, office, education, and residential buildings), industrial facilities (e.g.,
biotechnology, composting, waste disposal, manufacturing, textile, and food processing), and
outdoor environments (e.g., farms, feed lots, and general air quality). Monitoring for
bioaerosols in the occupational environment is one of the many tools the industrial hygienist
uses in the assessment of indoor air quality, infectious disease outbreaks, agricultural
exposures, and industrial health.
Bioaerosol monitoring may be appropriate during workplace health and exposure
assessments, epidemiological investigations, research studies, or in situations deemed
appropriate by an occupational physician or immunologist. Sampling can also be used to
evaluate occupational environments before and after mitigation of microbial contaminants.
When investigating bioaerosols as a possible source of workplace exposures and health issues,
bioaerosol sampling should be part of an integrated assessment of work conditions. This
should also include examining heating, ventilation and air conditioning (HVAC) systems;
checking for water infiltration and moisture control; evaluating microbial contamination in
evaporative cooling systems, metal working fluids, and waste water; evaluating possible

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-3 of BA-115
Sampling and Characterization of Bioaerosols
internal and external sources of bioaerosols; and other measures [Macher 1999]. In general, if
visible growth or contamination (microbial growth on floors, walls, or ceilings, or in the
HVAC system) is observed, this normally should be mitigated first before indoor bioaerosol
sampling is conducted. If personnel remain symptomatic after remediation, air sampling may
be appropriate, but the industrial hygienist should be aware that false negative results are
possible and should be interpreted with caution.
The industrial hygienist has a variety of tools and methodologies available to conduct an
environmental survey [ASTM 2014a; Flannigan et al. 2011; Hung et al. 2005]. However, many
of these approaches have lacked standardization and this has made the interpretation and
comparison between studies challenging [Flannigan et al. 2011]. In 2005, the American
Industrial Hygiene Association (AIHA) published the second edition of the Field Guide for
the Determination of Biological Contaminants in Environmental Samples [Hung et al. 2005].
This reference provides the industrial hygienist access to the most up to date methods to
detect and quantify bioaerosols in the environment, and covers methods of how to conduct a
survey, sample bioaerosols, and interpret the collected data [Hung et al. 2005]. Similarly, other
reference sources have been published by Flannigan et al. [2011] and the American
Conference of Governmental Industrial Hygienists (ACGIH) [Macher 1999] that extensively
outline available methods to analyze collected bioaerosols as well as strategies to conduct an
environmental survey. ASTM International has issued a wide range of standards on indoor air
quality, including assessment of fungal growth and collection of bioaerosols and a guide to
developing an air sampling strategy [ASTM 2009; ASTM 2014a; ASTM 2014b; ASTM 2014d].
The European Committee for Standardization has also published standards on sampling for
bioaerosols and related topics [CEN 2000; CEN 2003; CEN 2004]. The sections presented
below provide a very broad overview of the viable and non-viable methods available to detect
bioaerosol sources that are described in the references listed above.
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-4 of BA-115
Sampling and Characterization of Bioaerosols
Figure 1: Photomicrographs of acellular, prokaryotic and eukaryotic microorganisms that can
be encountered in occupational or industrial environments. (A) Transmission electron
micrograph of Influenza/flu (H1N1) virus particles (Photo courtesy of National Institute of
Allergy and Infectious Diseases; CDC Public Health Image Library (PHIL) ID#: 18156); (B)
Scanning electron micrograph of bacilli derived from the Gram-negative bacteria, Legionella
pneumophila (Photo courtesy of National Institute of Allergy and Infectious Diseases; CDC
Public Health Image Library (PHIL) ID#: 11150); (C) Scanning electron micrograph of
Aspergillus species reproductive structures including chains of asexual spores (Photo courtesy
of CDC/ Robert Simmons; CDC Public Health Image Library (PHIL) ID#: 13367); and (D)
Scanning electron micrograph of tricolpate pollen derived from the angiosperm plant species,
Oenothera fruticosa (Photo courtesy of CDC/ Janice Carr, Betsy Crane; CDC Public Health
Image Library (PHIL) ID#: 8729). The CDC Public Health Image Library at
http://phil.cdc.gov/Phil/home.asp has thousands of health-related images available to the
public free of charge.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-5 of BA-115
Sampling and Characterization of Bioaerosols
2 Principles of bioaerosol collection
a. Aerodynamic diameter
The aerodynamic diameter of an airborne particle (usually written as “da” or “dae”) is the
single most important parameter that determines how the particle will behave in the air,
including how long it will stay airborne and where it will deposit in the respiratory system
if inhaled. If a particle is falling in still air, it will reach an equilibrium velocity where the
gravitational force pulling it downward is balanced by the drag force on its surface. This
velocity is called the terminal settling velocity, and it depends upon the size, shape and
density of the particle. The aerodynamic diameter of a particle is defined as the diameter of
a sphere with unit density (that is, a density of 1 g/cm
3
) that has the same terminal settling
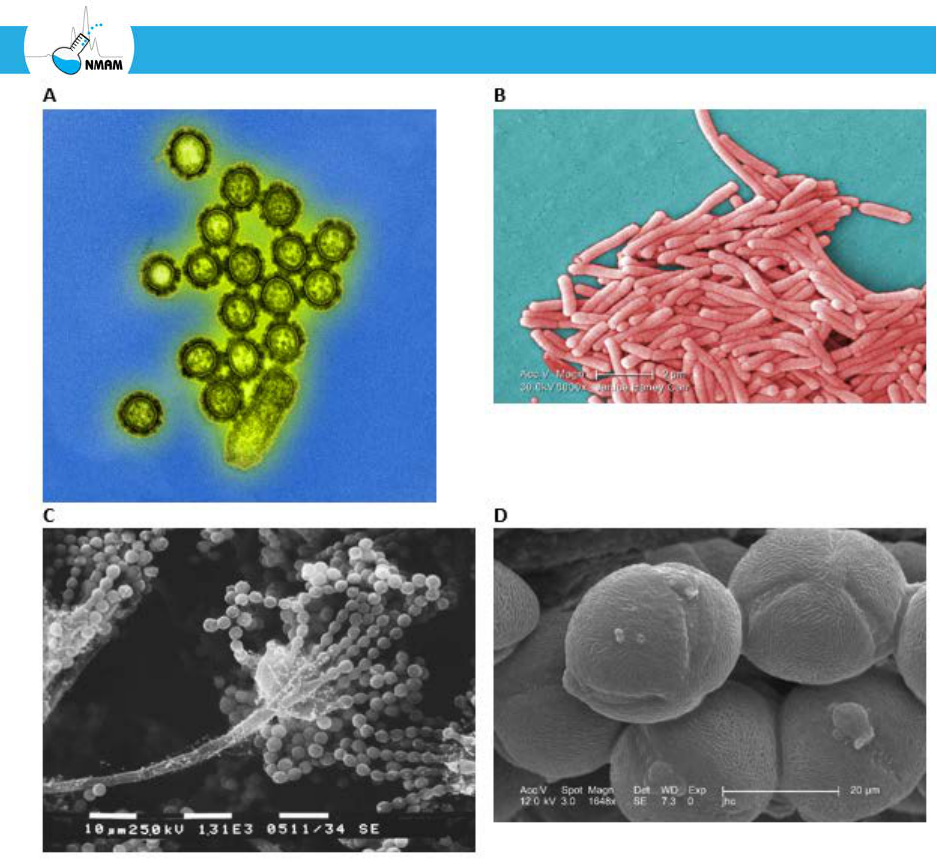
velocity as the particle. Consider, for example, the irregularly-shaped fungal fragment
shown in Figure 2. Suppose this particle has a terminal settling velocity of 0.05 cm/sec.
This is the same settling velocity as that of a spherical particle with a unit density that has a
diameter of 4 µm. Thus, the fungal fragment is said to have an aerodynamic diameter of 4
µm. Similarly, a different particle with a terminal settling velocity of 1.21 cm/sec has an
aerodynamic diameter of 20 µm, since a 20 µm unit density sphere settles at that rate. It is
important to note that the aerodynamic diameter may be very different from the physical
size of a particle. A very dense and compact particle may have an aerodynamic diameter
much larger than its actual dimensions, while a very light particle or one with fibrous
branches may have an aerodynamic diameter that is much smaller than its physical size. It
is possible for two particles to have very different shapes and physical sizes, but have the
same aerodynamic diameter. Conversely, two particles may have similar physical sizes, but
have very different aerodynamic diameters. A more detailed discussion of the
aerodynamic diameter can be found in Hinds [1999] and Vincent [2007].
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-6 of BA-115
Sampling and Characterization of Bioaerosols
Figure 2: Aerodynamic diameter of an aerosol particle. In this case, the fungal fragment on
the left is said to have an aerodynamic diameter of 4 µm, since it falls at the same terminal
settling velocity as a 4 µm sphere with a unit density.
Aerodynamic diameter is used in aerosol science because particles with the same
aerodynamic diameter tend to move and be collected in the same ways. For example, two
particles with the same aerodynamic diameter will have the same likelihood of being
collected by an impaction aerosol sampler even if they have different physical and
morphological characteristics. For this reason, the performance of aerosol collection
devices is usually described by giving the aerodynamic diameter of the particles that will be
collected.
b. Collection efficiency and cut-off diameter
The collection efficiency of an aerosol sampler is the fraction of the aerosol particles of a
particular aerodynamic diameter that will be collected by the sampler. For example, if 95%
of the airborne particles with a 2 µm aerodynamic diameter that enter the sampler are
deposited in the collection fluid or on the collection surface, then the sampler is said to
have a 95% collection efficiency for 2 µm particles.
Most commonly-used aerosol filters have a high collection efficiency for particles of all
sizes [NIOSH 2016b]. However, impactors, cyclones and impingers use the inertia of
airborne particles to separate them from the air stream, and thus they have a high
collection efficiency for particles with larger aerodynamic diameters and a low collection
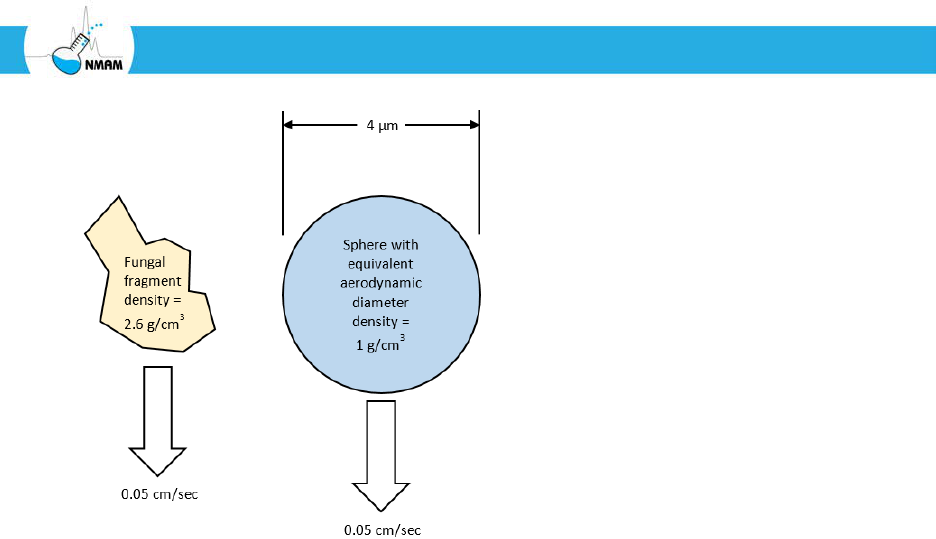
efficiency for smaller ones (Figure 3) [Hering 2001; Hinds 1999; Marple and Olson 2011].
These devices are said to have a “cut-off diameter”; that is, particles with an aerodynamic
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-7 of BA-115
Sampling and Characterization of Bioaerosols
diameter larger than the cut-off diameter are collected while particles with an aerodynamic
diameter less than the cut-off diameter are not collected and pass through the device. A
perfect collection device would have a 100% collection efficiency for particles larger than
the cut-off diameter and 0% for smaller particles. In practice, this is not the case: the
collection efficiency curve for an inertia-based sampler looks like the example curve shown
in Figure 3. The aerodynamic diameter at which the collection efficiency is 50% is defined
as the cut-off diameter (usually written as d
50
). A device with a more abrupt transition
from 100% to 0% collection efficiency (that is, closer to the ideal device) is said to have a
sharp cut-off.
For a given inertial collection device, the 50% cut-off diameter depends upon the air
flowrate through the device. Increasing the flowrate will decrease the d
50
and shift the
collection efficiency curve to the left, while decreasing the flowrate will increase the d
50
and
shift the collection efficiency curve to the right. For example, the first stage of the NIOSH
two-stage cyclone aerosol sampler has a d
50
of 4.9 µm at 2 liters/minute of air flow, 4.1 µm
at 3.5 liters/minute, and 2.1 µm at 10 liters/minute [Blachere et al. 2009]. For this reason, it
is important to check the air flowrate before aerosol sampling and control it during
sampling so that the particles are correctly segregated by size.
Figure 3: Example collection efficiency curve for an inertia-based aerosol sampler. Note
that the collection efficiency is high for particles with large aerodynamic diameters and
low for small particles. In this example, the 50% cut-off diameter (d
50
) for this device is 1
µm.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-8 of BA-115
Sampling and Characterization of Bioaerosols
c. Size-selective bioaerosol sampling in industrial hygiene
Size-selective bioaerosol sampling may be done for several reasons. Since the settling
velocity of aerosol particles is determined by the aerodynamic diameter, knowing the size
distribution of an aerosol helps in predicting how long the particles are likely to remain
airborne and how far they can travel. In health care settings, for example, various medical
procedures can produce a spray of droplets containing infectious microorganisms. Large
droplets tend to fall onto surfaces fairly close to the source, while smaller droplets can
remain airborne and carry pathogens many feet away from a patient [Davies et al. 2009;
Jones and Brosseau 2015]. Another application of size-selection is to isolate different types
of bioaerosol particles, such as separating fungal fragments from intact fungal spores
[Adhikari et al. 2013; Seo et al. 2014].
Size-selective sampling is most commonly used to help understand the potential health
effects of bioaerosol particles, which often depend upon where the particles are deposited
in the respiratory tract. In general, larger bioaerosol particles tend to deposit higher in the
respiratory tract (that is, in the nasal or oral cavities or larger airways), while smaller
particles are able to travel deeper into the lungs to the smaller airways [Hinds 1999;
Vincent 2005]. Some pathogens such as Mycobacterium spp., Bacillus spp., and Aspergillus
spp. are thought to be more likely to cause a pulmonary infection if they reach the deeper
airways, and the response to bioaerosols containing immunogenic material such as
endotoxins or fungal antigens may also vary depending upon the site of deposition. For
this reason, size-selective sampling is often used in industrial hygiene to better understand
the potential risks that workplace bioaerosols present.
The American Conference of Governmental Industrial Hygienists (ACGIH), the
International Organization for Standardization (ISO) and the European Standardization
Committee (CEN) have defined three particle collection efficiency curves for aerosol
samplers used to conduct size-selective aerosol sampling (Figure 4) [ACGIH 2001; ISO
2012; Vincent 2005]. The idea is that an aerosol sampler that conforms to one of the three
criteria will collect aerosol particles in a way that approximates the fraction of particles
that will reach different parts of the respiratory tract. These criteria are not specific to
bioaerosols, but rather are applied to all types of aerosol particles.
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-9 of BA-115
Sampling and Characterization of Bioaerosols
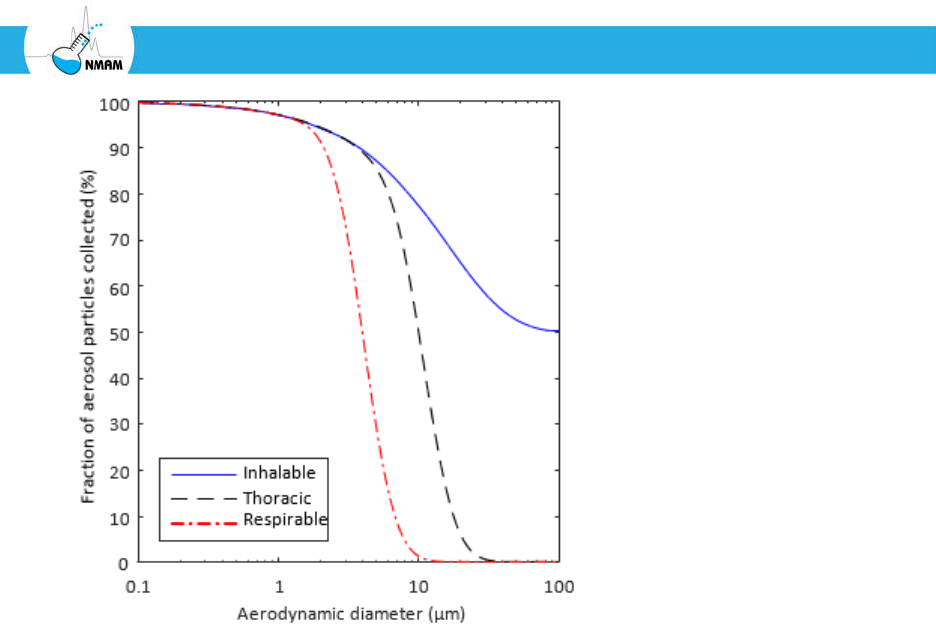
Figure 4: ACGIH/ISO sampling criteria for the inhalable, thoracic and respirable fractions
of aerosol particles. The inhalable fraction contains all of the particles that are inhalable,
which includes the particles in the thoracic and respirable fraction. Similarly, the thoracic
fraction includes the particles in the respirable fraction. The 50% cut-off diameters are 100
µm for the inhalable fraction, 10 µm for the thoracic fraction, and 4 µm for the respirable
fraction [ACGIH 2001; ISO 2012; Vincent 2005].
A sampler that collects the inhalable fraction accumulates the fraction of aerosol particles
of each size that would be expected to be drawn into the nose or mouth during normal
breathing. This includes larger particles that would be expected to be deposited in the
nasal or oral cavities as well as smaller particles that can be conveyed to the lower airways.
An aerosol sampler that conforms to the inhalable sampling criteria collects 50% of the
100 µm particles, 77% of the 10 µm particles, and 97% of the 1 µm particles in the ambient
aerosol. The inhalable fraction is lower for larger particles because the greater inertia of
these particles means they are less likely to be pulled into the body during inhalation.
The thoracic fraction includes aerosol particles that are likely to travel into the trachea and
bronchi. An aerosol sampler that conforms to the thoracic sampling criteria will collect
50% of the 10 µm particles and 97% of the 1 µm particles in the ambient aerosol. This
fraction includes fewer large particles because these particles tend to be removed from the
airstream by the head airways.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-10 of BA-115
Sampling and Characterization of Bioaerosols
The respirable fraction includes aerosol particles that are able to reach the deepest airways,
which are the respiratory bronchioles and the alveoli. An aerosol sampler that conforms to
the respirable sampling criteria will collect 50% of the 4 µm particles, 97% of the 1 µm
particles, and 99% of the 0.3 µm particles in the ambient aerosol. The respiratory
bronchioles and the alveoli are of particular concern because these airways do not have
cilia. Non-soluble particles that land in the nasopharyngeal region or upper airways tend
to collect in the airway mucus and are removed from the respiratory tract by the cilia
relatively quickly. However, particles that deposit in the alveoli and respiratory
bronchioles can remain in the lungs for longer durations (in some cases, for life) unless
they can be broken down or removed by migrating pulmonary macrophages. This fraction
includes only the smallest particles because the larger particles are removed from the
airstream by the head and thoracic airways.
It should be noted that, even though larger bioaerosol particles will tend to deposit in the
upper airways and be cleared more quickly, they can still trigger an allergic/inflammatory
response in susceptible individuals. Particles containing viable pathogens also commonly
cause infections after being deposited in the upper airways.
When describing size-selective sampling, particles are often said to “penetrate” to a
particular region of the respiratory tract. This does not mean penetrate in the sense of
entering the tissue, but rather simply being present in the air stream flowing into that
region, as compared to particles which were deposited before reaching a particular
location. For example, an aerosol particle that is able to remain in the air stream and reach
the lung alveoli is said to have penetrated to the alveolar region, even if it does not
necessarily deposit there. This is the same context as with filtration, where a particle is said
to penetrate a filter if it flows through the filter material and remains in the air stream. It
also should be noted that the ACGIH/ISO criteria give an approximation of the fraction of
aerosol particles that can penetrate to different regions of the respiratory tract. However,
they do not indicate what fraction of the aerosol particles will actually deposit in the
airways and what fraction will be exhaled. The lung deposition of aerosol particles is
complex and depends upon many factors. More information about this topic can be found
in Hinds [1999] and Vincent [2005; 2007].
3 Devices used for bioaerosol sampling
Most aerosol sampling devices involve techniques that separate particles from the air stream
and collect them in or on a preselected medium. Impactors, filters, impingers and cyclones are
four common sampling techniques used to separate and collect bioaerosols [Haig et al. 2016;
Macher et al. 1995; Reponen et al. 2011b; Willeke and Macher 1999]. A few systems that use
electrostatic precipitation or condensation-based collection are also available [Haig et al.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-11 of BA-115
Sampling and Characterization of Bioaerosols
2016], and some real-time bioaerosol monitoring systems are available that do not require
that the bioaerosol particles be isolated before analysis. Below are some specific types of
bioaerosol sampling devices employed by industrial hygienists.
a. Filters
Aerosol filters are commonly used to collect bioaerosol particles because of their simplicity
and low cost. Filter-based sampling is particularly useful for personal bioaerosol sampling
because filter-based collectors are small and lightweight and work well with personal
sampling pumps. Filters can be preceded by a size-selective inlet, such as a cyclone or
impactor, to remove larger particles and provide size-classification of the bioaerosol
particles. Most aerosol filter media can be classified as fibrous, membrane, or capillary
pore (also called straight-through pore) [Raynor et al. 2011]. Fibrous filters are usually
made of a deep mesh of glass fibers. Membrane filters are manufactured in a variety of
pore sizes from polymers such as cellulose ester, polyvinyl chloride, or
polytetrafluoroethylene (PTFE). Capillary pore filters are made of polycarbonate. The
choice of a filter medium depends on the contaminant of interest and the requirements of
the analytical technique. For gravimetric analysis, non-hygroscopic materials such as glass
fibers, silver, or polyvinyl chloride membranes are selected because their masses are less
affected by changes in humidity. For analysis by microscopy, cellulose ester or
polycarbonate membranes are common choices because cellulose ester membranes can be
rendered transparent for easier visualization, while polycarbonate filters have a smooth
collection surface that works well with light or electron microscopy. Samples also can be
eluted from cellulose ester and polycarbonate filters, but in some cases the recovery
efficiency can be low [Eduard et al. 1990; Rule et al. 2007]. Samples to be cultured can be
collected on gelatin filters, and the filters can then be dissolved in water and spread on
culture plates, dissolved in growth media, or placed directly on culture plates and allowed
to melt. Gelatin filters are fragile and can crack or melt in use. For analysis using
immunological assays or polymerase chain reaction (PCR), PTFE filters are a common
choice because they do not interfere with the assays and because samples can be readily
eluted from them.
Filters are frequently described or specified using the term “pore size” or “equivalent pore
diameter”. It is important to note that the filter pore size does NOT indicate the minimum
particle size that will be collected by the filter; in fact, aerosol filters generally will collect
particles much smaller than the nominal pore size. The mechanisms by which aerosol
filters work and the role of pore size in selecting filters is described is more detail
elsewhere [NIOSH 2016b].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-12 of BA-115
Sampling and Characterization of Bioaerosols
Aerosol filters are usually supplied as disks of 25, 37 or 47-mm diameter. Because the flow
resistance (often called the pressure drop) of a filter increases with the air velocity through
the filter, the use of a larger filter results in a lower flow resistance for a given volumetric
flow rate. On the other hand, the use of a smaller filter concentrates the deposit of the
contaminant onto a smaller total area, thus increasing the density of particles per unit area
of filter. This may be helpful for direct microscopic examination of low concentrations of
organisms, and reduces the amount of elution media needed for immunological or PCR-
based assays. In areas of high concentration, the microorganisms may have to be eluted,
diluted, and then refiltered for microscopic analysis. Breuer [2012] reported on the flow
resistance of common aerosol filters and its relationship to sampling pump selection. Soo
et al. [2016] measured the filtration characteristics and flow resistance of a variety of
commonly-used aerosol filters.
In the USA, the most common method of aerosol sampling with filters is to place the
filters in disposable two-piece or three-piece plastic filter cassettes with a support pad to
add rigidity. The three-piece cassette may be used either in open- or closed-face modes.
Open-face sampling is performed by removing the end plug and the plastic cover from the
three-piece cassette and is used when the particulate matter must be uniformly deposited
(i.e., for microscopic analysis). If a three-piece cassette is used in the open-face
arrangement, the plastic cover is retained to protect the filter after sampling is concluded.
It should be noted that the aspiration efficiencies of open-face and closed-face filter
cassettes are reported to be somewhat different [Beaulieu et al. 1980; Kenny et al. 1997].
In addition to collecting on the filter, aerosol particles (especially large particles) may
collect on the internal walls of the filter cassette. Depending upon the purpose of the
collection, wall-deposited material may need to be included in the analysis. This can be
done by using a filter with an attached capsule or by washing or wiping the internal
surfaces of the cassette [Ashley and Harper 2013].
It is important to verify that the filter cassette and fittings are air-tight and have no bypass
leakage around the filter. Cassettes should not be hand-assembled; they should be pressed
together with a mechanical or hydraulic press. All plastic cassettes should be securely
assembled and sealed with a cellulose shrink band or tape around the seams of the cassette
to prevent external air leakage. The cassettes should be made of conductive or static
dissipative materials to avoid losses due to electrostatic effects. More information on using
filter cassettes for aerosol sampling can be found elsewhere [NIOSH 2003a; NIOSH
2016a].
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-13 of BA-115
Sampling and Characterization of Bioaerosols
b. Impactors
An impactor consists of a series of nozzles (circular- or slot-shaped) and an impaction
surface [Hering 2001; Marple and Olson 2011; Marple and Willeke 1976]. Air is drawn
into the impactor using a vacuum pump, and the air stream flows through the nozzles and
toward the impaction surface, where particles are separated from the air stream by their
inertia (Figure 5). Larger particles collect on the impaction surface, while small particles
that do not impact follow the air stream. The impaction surface typically consists of a
greased plate or tape, filter material, or growth media (agar) contained in Petri dishes. In
some applications, impactors are not used as collection devices themselves, but rather to
remove particles above a certain size before collection or characterization of the
downstream aerosol.
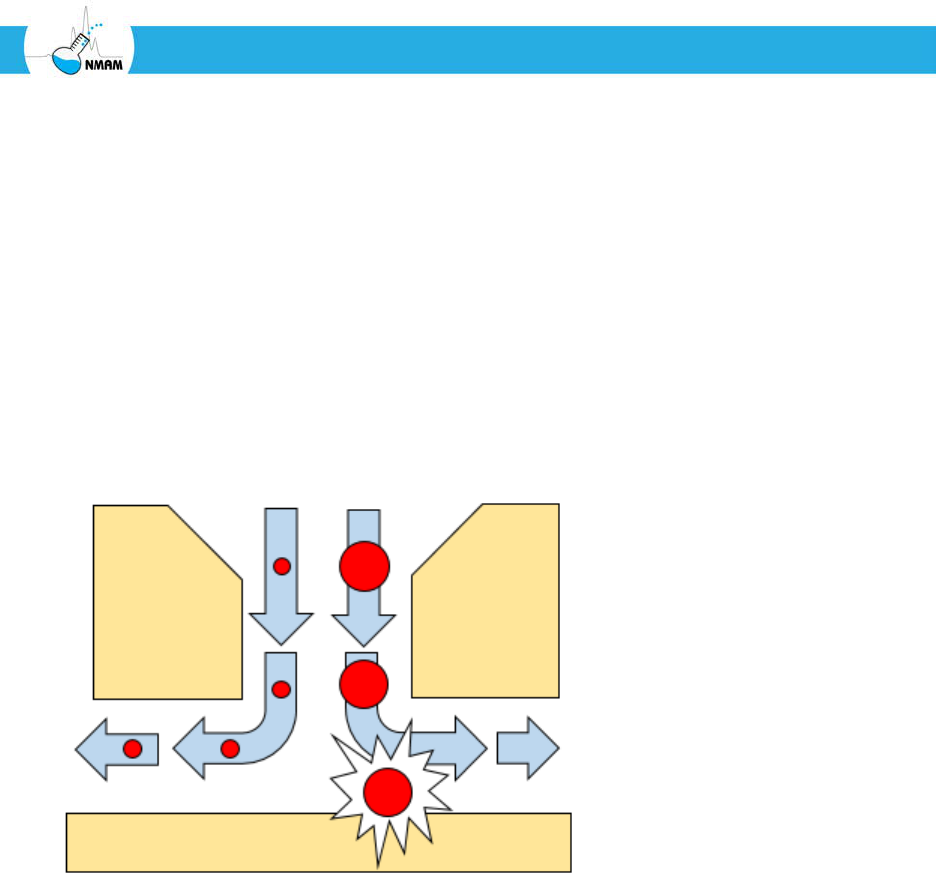
Figure 5: Impaction. As the air stream exits the impactor nozzle, it quickly changes
direction as shown by the arrows. Smaller particles such as those on the left flow with the
air stream and are not collected. Larger particles cannot change direction as quickly due to
their higher inertia and collide with the collection surface, where they accumulate.
A cascade impactor consists of a stack of impaction stages: each stage consists of one or
more nozzles and a target or substrate. The nozzles may take the form of holes or slots.
Each succeeding stage has smaller nozzles and thus collects smaller particles (that is, each
succeeding stage has a smaller cut-off diameter). A filter may be used after the final
impaction stage to collect any particles smaller than the final cut-off diameter. If the
substrate is a greased plate or filter media, it may be weighed to determine the collected
mass, or it may be washed and the wash solution analyzed. If the substrate is growth media
in culture plates, they may be incubated and examined for microbial growth.
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-14 of BA-115
Sampling and Characterization of Bioaerosols
The most commonly used impactor for sampling airborne culturable bacteria and fungi is
the Andersen impactor, which uses from one to six impactor stages containing Petri plates
as seen in Figure 6 [Andersen 1958]. Since the bioaerosol particles impact directly onto the
growth media, the samplers can be directly transferred to an incubator and observed for
microbial growth. However, this method depends upon collecting viable microorganisms
that are capable of growth on the specific nutrient media.
Glass Petri plates are recommended for use with the Andersen impactor; plastic culture
plates are often used, but this can result in loss of aerosol material due to electrostatic
surface charges in the plastic [Andersen 1958; Kuo 2015]. NIOSH Method 0800 describes
how to collect culturable airborne fungi and bacteria in buildings using an Andersen
cascade impactor [NIOSH 2003b].
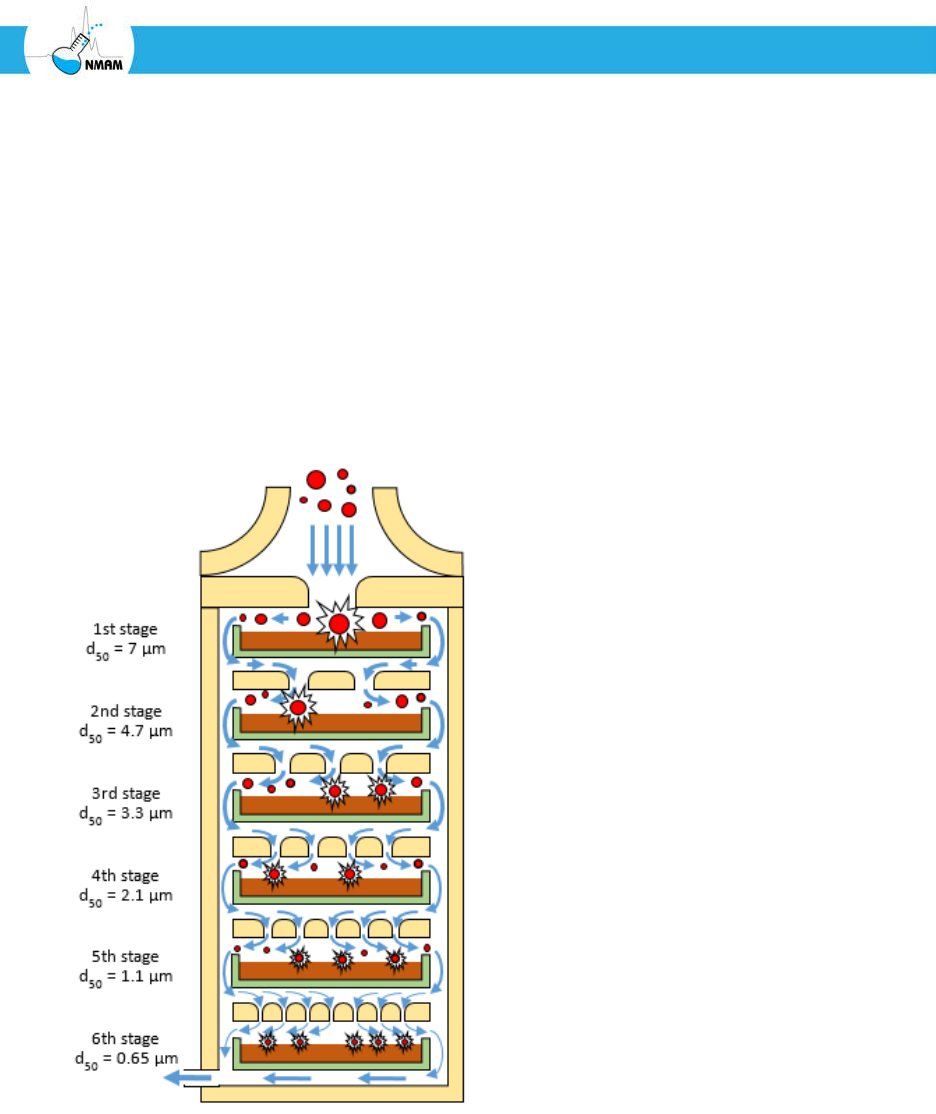
Figure 6: Schematic of a 6-stage Andersen cascade impactor [Andersen 1958]. Each stage
contains a Petri plate (green) filled with nutrient agar (brown). The stages have
progressively smaller nozzles, which create higher particle impaction velocities onto the
agar. The aerosol particles (red) flow from the top into the first stage, where particles with
aerodynamic diameters larger than 7 µm impact the agar. The remaining particles flow to
the second stage, where particles with aerodynamic diameters between 7 µm and 4.7 µm
are collected, and so on for the rest of the stages.
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-15 of BA-115
Sampling and Characterization of Bioaerosols
One significant advantage of the Andersen impactor is that samples can be collected
directly onto culture plates and transferred to an incubator, which simplifies handling and
eliminates some losses that can occur in processing. However, there are also several
limitations. In low concentration environments, sampling time is limited to approximately
20 minutes to avoid drying the agar. The high flow rate (28.3 liters/minute) makes the
sampler unsuitable for high concentration environments such as some agricultural sites
(i.e. animal facilities) where a 1 minute sample may overwhelm the plates.
When using the Andersen impactor, it is also necessary to correct for “coincidence error”
using a positive-hole correction factor. This occurs because it is possible for multiple
particles, each containing one or more organisms, to pass through a particular hole during
sampling and impact onto the growth medium, with one or more bacterial or fungal
colonies forming at the same impaction sites. The colonies formed by the multiple
particles can then be inaccurately counted as a single colony. As the number of organism-
containing particles deposited onto the growth medium increases, the probability that the
next organism-containing particle will impact an "occupied" hole increases. For example, if
75% of the holes have received at least one particle, the chance that the next particle will
impact a "clean" hole is one in four (25%). To account for this, a probability-based
coincidence correction factor needs to be applied to the results for each impactor stage.
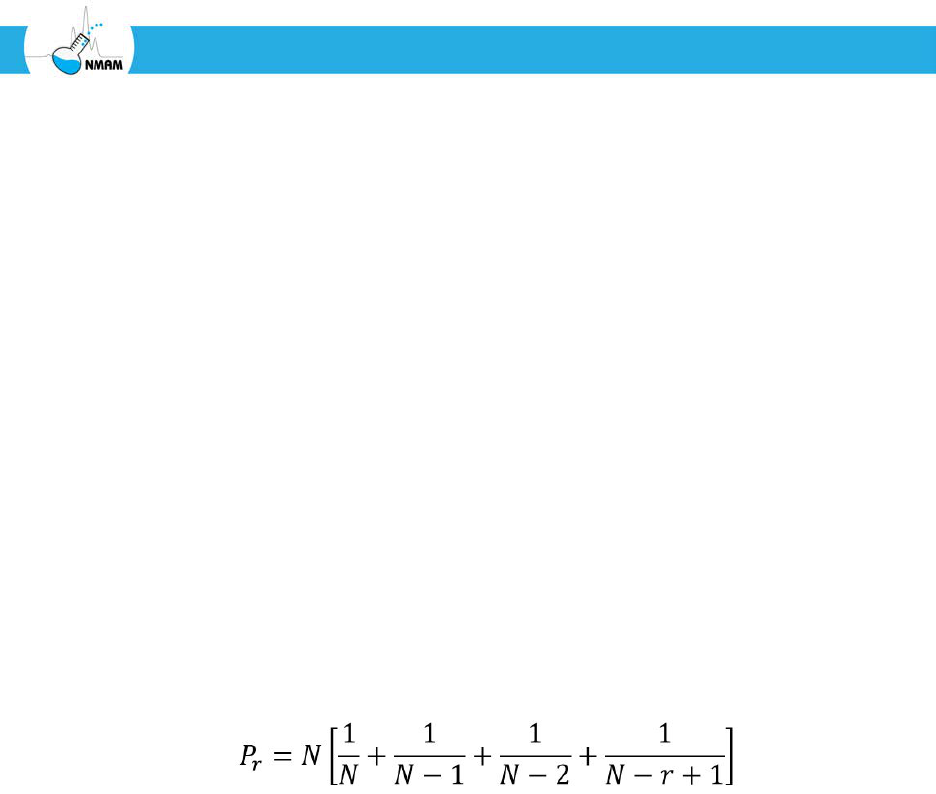
The basic formula for the coincidence correction is as follows [Andersen 1958; Macher
1989]:
Where:
N = the total number of holes in the impactor stage
r = the number of colonies observed on the culture plate
P
r
= the estimated culturable particle count
Andersen impactors have from one to 400 holes per stage. Macher [1989], Willeke and
Macher [1999] and Andersen [1958] provide tables of positive-hole correction factors.
Investigators often employ stationary cascade impactors either as the primary collection
mechanism, or as a preclassifier (for example, to remove nonrespirable particles from the
sampled air stream). Marple and Willeke [1976] have reported that high velocity, inlet
losses, interstage losses, and particle reentrainment affect the performance characteristics
of an impactor. Particles larger than the cut-off diameter may bounce after impacting the
collection surface and travel to subsequent impaction stages. This is particularly a problem
with dry solid collection surfaces; for this reason, solid collection surfaces are usually
greased or oiled [Hering 2001]. Fungal spores have been shown to be prone to de-
aggregation and bounce when collected with an impactor, which can cause the spores to be
collected on stages with smaller cut-off diameters. This can make the spore aggregates
appear to have smaller aerodynamic diameters than is actually the case [Trunov et al.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-16 of BA-115
Sampling and Characterization of Bioaerosols
2001]. Although personal cascade impactors are available, these devices are not as widely
used in personal sampling for bioaerosols as are filters [Macher and Hansson 1987].
The slit-to-agar impactor is a type of impactor in which the aerosol particles are deposited
on a Petri plate that slowly rotates. The rotation of the plate means that particles which are
collected at different times deposit in different locations, and thus provides an indication
of changes in the bioaerosol concentration over time [Ho et al. 2005; Jensen et al. 1992;
Smid et al. 1989; USP 1997]. Examples of slit-to-agar samplers include the Dycor Slit
Sampler from Dycor and the Air Trace Environmental Slit-to-Agar Sampler from Particle
Measuring Systems.
The Hirst/Burkard spore trap has been widely used to collect outdoor aerospora. It was
first described by Hirst [1952] and consists of a unit that houses a vacuum pump and
rotating drum that is lined with polyester tape. The drum rotates at 2 mm per hour and is
continuously run for seven days. Bioaerosols pass through an orifice on the sampler and
particles impact on the tape. Following the seven-day sampling interval, the tape is
removed and cut in 48 mm intervals that correspond to individual sampling days.
Bioaerosols deposited on the tape are stained and then resolved, identified, and quantified
using bright field microscopy.
[Tovey et al. 2016] developed a personal aerosol sampler with a rotating surface that allows
time-resolved collection of aerosol particles onto an electret strip or an adhesive film. They
used the sampler to study personal exposures to dust mite allergens over time.
A novel example of an impaction-based personal bioaerosol sampler is the intranasal air
sampler fabricated by Graham et al. [2000], which fits within the intranasal cavity of the
subject. Bioaerosols enter the nasal cavity following inhalation and pass through slits
where particles are deposited by impaction on either an adhesive backed tape or collection
cup lined with silicon grease. This impaction sampler has been utilized in a number of
studies that have evaluated exposure to indoor and occupationally relevant aeroallergen
sources [Gore et al. 2002; Mitakakis et al. 2000; Renstrom et al. 2002].
Other impaction-based approaches have also been used in the assessment of outdoor
bioaerosols, including the Rotorod, Air-o-cell and Allergenco samplers [Frenz 1999; Lee et
al. 2004a; Pityn and Anderson 2013; Portnoy et al. 2000]. ASTM Standards D7391 and
D7788 discuss the collection and analysis of airborne fungal structures by inertial
impaction [ASTM 2009; ASTM 2014d].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-17 of BA-115
Sampling and Characterization of Bioaerosols
c. Cyclones
A cyclone sampler consists of a circular chamber with the aerosol stream entering through
one or more tangential nozzles as shown in Figure 7 [Hering 2001]. Like an impactor, a
cyclone sampler depends upon the inertia of the particle to cause it to deposit on the
sampler wall as the air stream curves around inside the chamber. Also like an impactor, a
cyclone sampler has a collection efficiency curve like the one shown in Figure 3, and the
collection efficiency curve depends upon the flow rate. Cyclones are less prone to particle
bounce than impactors and can collect larger quantities of material. They also may provide
a more gentle collection than impactors, which can improve the recovery of viable
microorganisms. However, cyclones tend to have collection efficiency curves that are less
sharp than impactors, and it is simpler to design a compact cascade impactor compared to
a cascade of cyclone samplers.
In industrial hygiene, cyclone aerosol samplers are frequently used in conjunction with a
filter to conduct size-selective aerosol sampling [Hering 2001]. For example, in NIOSH
Method 0600, a cyclone is used to remove the non-respirable fraction from the aerosol
(following the ACGIH/ISO criteria described earlier), and a filter is then used to collect the
respirable fraction [NIOSH 2003c]. A sampler developed at NIOSH uses two cyclones
followed by a filter; the first cyclone collects the non-respirable fraction of the particles, the
second cyclone collects the respirable particles > 1 µm, and the filter collects particles < 1
µm [Blachere et al. 2009]. The NIOSH cyclone aerosol samplers have been used in
applications including measurements of airborne viruses in healthcare settings; airborne
fungi and fungal fragments in residences; airborne dimorphic fungal pathogens such as
Paracoccidioides brasiliensis in Brazil, and bioaerosols in agricultural operations [Arantes
et al. 2013; Blachere et al. 2009; Blais Lecours et al. 2012; Kettleson et al. 2013; Lee and Liao
2014; Lindsley et al. 2010a; Lindsley et al. 2010b; Martin et al. 2015; Seo et al. 2014; Singh
et al. 2011a; Singh et al. 2011b].
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-18 of BA-115
Sampling and Characterization of Bioaerosols
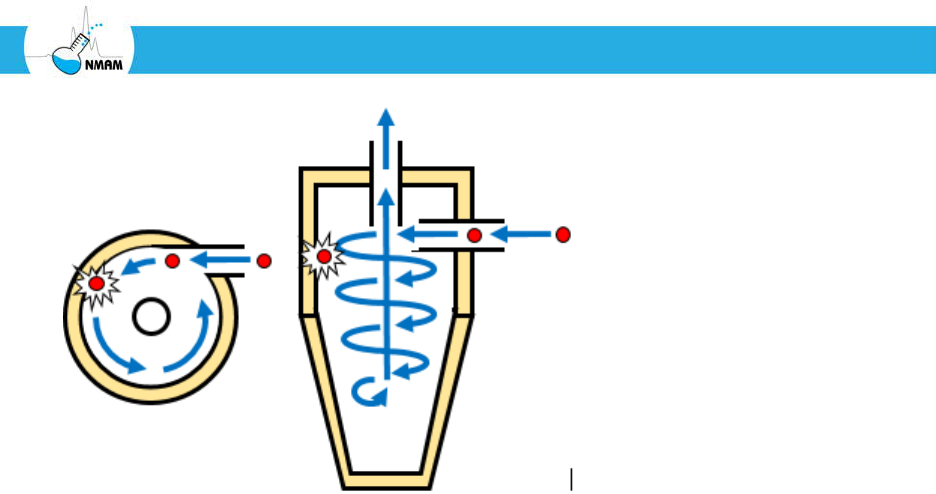
Figure 7: Cyclone aerosol collection. When the aerosol stream enters the body of the
cyclone through the inlet, the air flow follows the curved interior wall and flows in a spiral
pattern. If aerosol particles are larger than the cut-off diameter, then the inertia of the
particles causes them to collide with the wall of the cyclone and accumulate. After
spiraling downward, the air flow comes up through the center of the cyclone and exits
through the outlet (called a vortex finder) at the top. The illustration shows a tangential
inlet reversed-flow cyclone, which is the most common type of cyclone sampler.
d. Impingers
Many microorganisms can lose their viability if they are collected onto dry solid surfaces
or filters because of impact damage and desiccation [Cox 1987; Jensen et al. 1992; Macher
and First 1984; Verreault et al. 2008; Wang et al. 2001]. One way to avoid this is to collect
culturable bioaerosols in liquids using an impinger [Henningson and Ahlberg 1994;
Henningson et al. 1988; Lembke et al. 1981; Reponen et al. 2011b; Verreault et al. 2008]. A
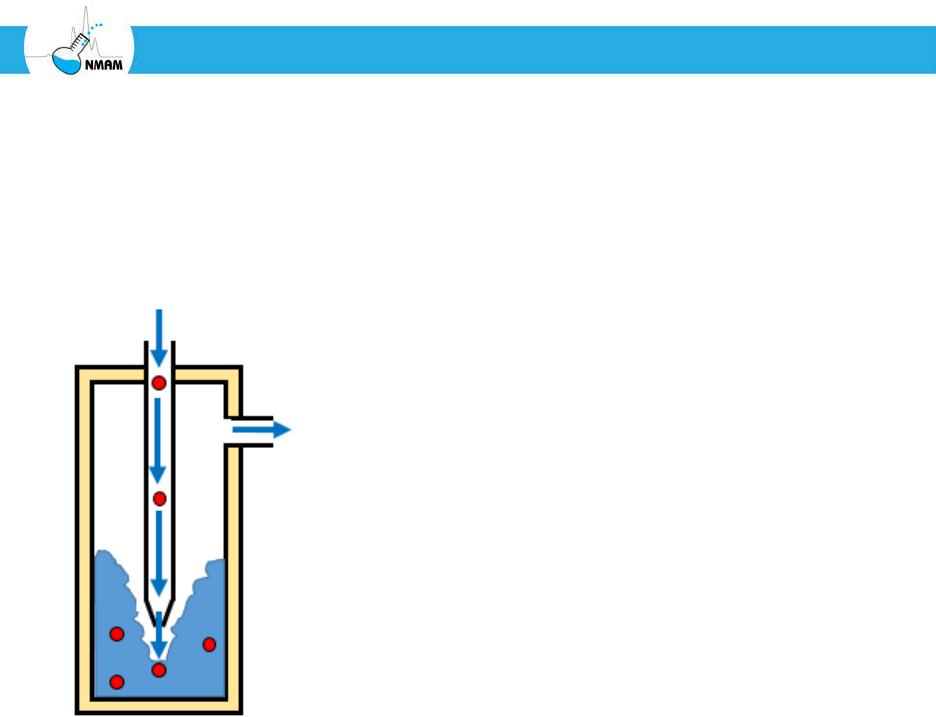
typical impinger is shown in Figure 8. The body of the impinger is filled with a collection
liquid, and the aerosol stream flows down through a nozzle and enters the liquid at a high
velocity. The aerosol particles are collected when they collide with the bottom of the
collection vessel or disperse into the liquid. Impingers often have curved inlets to remove
larger particles from the air stream before collection. Because impingers are essentially
another type of inertial collection device, they have a collection efficiency curve and a cut-
off diameter like impactors and cyclones. However, the collection efficiency curves tend to
be less sharp. The high velocity air stream directed into the liquid also creates considerable
agitation and can produce foaming if the collection liquid contains surfactants. Additives
to the collection medium such as proteins, antifoam, or antifreeze aid in resuscitation of
bacterial cells, prevent foaming and loss of the collection fluid, and minimize injury to the
cells [Chang and Chou 2011; Cown et al. 1957; Dungan and Leytem 2015]. The presence
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-19 of BA-115
Sampling and Characterization of Bioaerosols
of proteins and other additives can also greatly influence the survival of airborne viruses
during collection by impingers [Ijaz et al. 1985b; Schaffer et al. 1976; Verreault et al. 2008].
Water loss over time reduces the liquid level in the impinger and increases the
concentration of the non-volatile components, which limits the available collection time
[Lin et al. 1997]. Sample losses due to re-aerosolization and particle deposition inside the
impinger can be significant [Grinshpun et al. 1997; Han and Mainelis 2012].
Figure 8: Impingement. Bioaerosol particles exit the nozzle of the impinger at high velocity
and impact the liquid or the bottom surface of the collection vessel. Some types of
impingers produce air bubbles in the collection media, which can enhance particle
collection, but can damage some types of microorganisms.
Two common impingers used for bioaerosol sampling are the Greenburg-Smith impinger
[Greenburg 1932] and the All-Glass Impinger with the nozzle 30 mm above the base of the
collection vessel, called the AGI-30 [May and Harper 1957]. The Greenberg-Smith and
AGI-30 samplers operate by drawing aerosols at nominal flow rates of 28.3 and 12.5
L/min, respectively, through an inlet tube [Macher et al. 1995]. The AGI-30 inlet tube is
curved to simulate particle collection in the nasal passage [Cox 1987]. Investigators have
reported problems with low sampling efficiencies and high losses due to particles in the
collection being re-aerosolized and lost [Grinshpun et al. 1997; Kesavan et al. 2010; Lin et
al. 1997].
When the AGI-30 is used to recover total airborne organisms from the environment, the
curved inlet tube is washed with a known amount of collecting fluid after sampling
because larger particles (i.e., over 15 µm) are collected on the tube wall by inertial force.
After sampling for the appropriate amount of time, 10 mL of the full-strength collection

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-20 of BA-115
Sampling and Characterization of Bioaerosols
fluid is filtered through a 0.45-µm pore size membrane filter. Serial dilutions of the
remaining collection fluid are handled similarly [Greenberg et al. 1992]. The membrane
filters are placed in sterile plastic petri plates filled with the appropriate medium and
incubated for later identification and enumeration.
e. Wetted-surface bioaerosol samplers
Several types of bioaerosol sampling devices have been developed in which the aerosol
stream impacts onto a wetted surface or onto the wall of a cyclone wetted with collection
media [Kesavan and Sagripanti 2015; Kesavan et al. 2011]. These systems largely avoid the
bubbling and agitation associated with conventional impingers, which may be detrimental
to some microorganisms [Lin et al. 2000], and can provide sharper collection efficiency
curves. One of the simplest examples of a wetted-surface sampler is the SKC BioSampler
[Lin et al. 2000; Willeke et al. 1998]. It is similar to an AGI-30, except that it has three
nozzles that curve so that the aerosol stream is tangential to the wall of the collection
vessel. This causes the collection liquid to swirl and greatly reduces the agitation, bubbling
and consequent reentrainment seen with the AGI-30. The BioSampler collects particles
with aerodynamic diameters of approximately 0.3 µm to 8 µm into the collection media,
although the upper cut-off diameter is not sharp [Hogan et al. 2005; Kesavan et al. 2010;
Willeke et al. 1998]. The BioSampler reportedly can be used with non-evaporating fluids
such as mineral oil to eliminate the collection time limits imposed by water evaporation,
provided that the microorganism can survive collection and processing [Lin et al. 2000].
Alternatively, fluid can be exchanged or added to the sampler as needed [Rule et al. 2005;
Rule et al. 2007].
The CIP10-M, a modified version of the CIP10 aerosol sampler, collects airborne
microorganisms in a liquid layer on the interior surface of a rapidly-rotating cup. As with
the BioSampler, the CIP10-M can be used with mineral oil as the collection fluid to avoid
fluid evaporation. It is reported to have collection efficiencies of >80% for particles >2.8
µm, 50% for 2.1 µm particles, and <10% for particles of <1 µm [Görner et al. 2006; Simon
et al. 2016].
May [1966] designed a three-stage sampler in which aerosol particles are collected by
impaction onto a wetted fritted surface in the first two stages and the third stage is a
swirling aerosol collector similar to the BioSampler. Both glass and stainless steel versions
are available. In his original report, May [1966] used particles with a density of 1.5 g/cm
3
and reported cut-off sizes of 6 µm, 3.3 µm and 0.7 µm, which correspond to aerodynamic
diameters of about 7.3 µm, 4 µm, and 0.86 µm. The May sampler is reported to give
comparable results to the Andersen impactor [Zimmerman et al. 1987].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-21 of BA-115
Sampling and Characterization of Bioaerosols
Several wetted-surface bioaerosol samplers recirculate the collection fluid and add
additional fluid as needed to replace evaporative losses. This extends the collection time
available and allows the concentration of the aerosol from a large volume of air at a high
flow rate into a relatively small volume of liquid, which is of great advantage when
searching for pathogens that may be present in very low concentrations. For this reason,
such systems are often used for bioterrorism and homeland security applications. The
Coriolis sampler [Carvalho et al. 2008], the OMNI-3000 [Zhao et al. 2014], the SASS 2000
[Ravva et al. 2012], and the SpinCon [Yooseph et al. 2013] use a wetted wall cyclone for
bioaerosol collection, while the BioCapture 650 [Ryan et al. 2009] collects particles onto a
wetted rotating impactor. Kesavan and Sagripanti [2015] reported the results of
performance tests for several of these types of bioaerosol samplers.
When conducting long-term bioaerosol collection into liquid media, it is important to
note that if the collected bioaerosol particles remain in the collection media for an
extended time and if steps are not taken to inhibit growth, spore germination and cell
amplification of some fungi and bacteria can occur. This can result in the appearance of
much higher bioaerosol concentrations than are actually present in the environment.
f. Condensation-based bioaerosol samplers
Some bioaerosol particles are too small to be readily collected by impactors or impingers.
These particles can be collected using filters, but filter collection can reduce the viability of
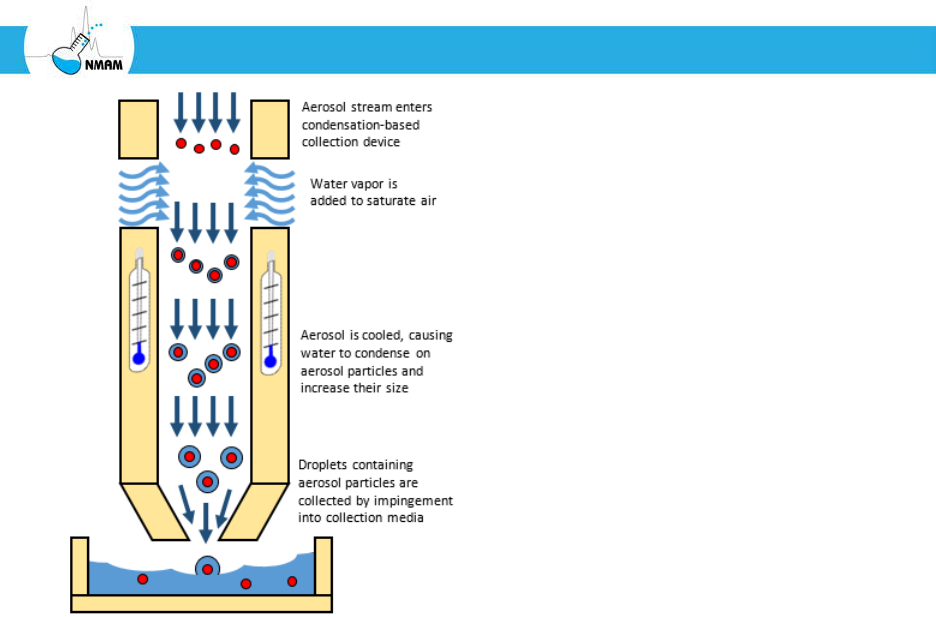
microorganisms. One solution is to humidify the aerosol stream and then cool it, which
causes water vapor to condense on the aerosol particles and create a droplet surrounding
the particle. This larger particle can then be collected by impaction or impingement, as
shown in Figure 9. This is similar in principle to condensation-based particle counters,
which are used to measure the concentration of small airborne particles. Some researchers
showed that adding water vapor to an aerosol stream enhanced the recovery of airborne
viruses and bacteriophages, which may work by this method (although this is unclear)
[Hatch and Warren 1969; Trouwborst and Kuyper 1974; Warren et al. 1969]. More
recently, Milton developed a condensation-based system to collect fine particles
containing influenza virus from the exhaled breath of human subjects [McDevitt et al.
2013; Milton et al. 2013]. A condensation-based bioaerosol sampler called a growth-tube
collector has been used to collect MS2 bacteriophage and influenza virus in the laboratory,
and is reported to be especially effective at recovering viable virus in sub-micrometer
particles [Lednicky et al. 2016; Pan et al. 2016; Walls et al. 2016]. A version of this system
called the Spot Sampler (Aerosol Devices, Inc.) is commercially available.
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-22 of BA-115
Sampling and Characterization of Bioaerosols
Figure 9: Condensation-based aerosol particle collector.
g. Electrostatic samplers
Electrostatic precipitation works by using a strong electric field to create a high
concentration of unipolar ions. The rapid motion of these ions causes them to collide with
and charge airborne particles, and the resulting charge on the particles causes them to be
attracted to the collection surface [Hinds 1999]. Electrostatic precipitation systems have
been used to collect bioaerosol particles such as allergens, bacteria and viruses [Artenstein
et al. 1968; Artenstein et al. 1967; Custis et al. 2003; Donaldson et al. 1982; Heitkamp et al.
2006; Lee et al. 2004b; Parvaneh et al. 2000; Roux et al. 2013]. Such devices offer simplicity
of design with few moving parts, and are generally effective at collecting small particles.
One electrostatic bioaerosol sampling device is available commercially from Inspirotec
[Gordon et al. 2015].
Some electrostatic bioaerosol samplers collect particles into liquid to concentrate the
particles and help preserve the viability of microorganisms. The Large Volume Air
Sampler (LVS) developed by Litton in the 1960’s washed the collection surface with
recirculating fluid; this sampler was successfully used to collect pathogenic respiratory
bacteria and viruses in a variety of settings [Artenstein et al. 1968; Artenstein et al. 1967;
Donaldson et al. 1982]. Pardon et al. [2015] developed a system that collects particles
directly on a microfluidic chip. The electrostatic aerosol collector devised by Han et al.
[2015] collects the deposited aerosol into rolling water droplets, which greatly

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-23 of BA-115
Sampling and Characterization of Bioaerosols
concentrates the particles. The Aerosol-to-Liquid Particle Extraction System (ALPES) uses
an electrostatic system to collect aerosol particles into recirculating liquid, which helps
preserve the viability of microorganisms [Heitkamp et al. 2006].
Electrostatically-charged cloths are used to collect airborne particles that settle onto them,
and also to wipe settled dust from surfaces. These are discussed in the next two sections.
h. Passive bioaerosol samplers
Passive bioaerosol sampling refers to the collection of bioaerosols by allowing them to
gravitationally settle onto a collection device, such as a culture plate, foil sheet, electret-
based filter or electrostatically-charged cloth. Compared to active sampling, passive
bioaerosol sampling has several advantages, including simplicity, low cost, lack of
disturbance of the surrounding air, and the ability to collect for extended time periods
[Haig et al. 2016; Pasquarella et al. 2000; Vincent 2007].
Passive bioaerosol sampling can be limited by several variables including the air currents
around the device and airborne particle size. As discussed earlier, large particles settle
much more quickly than small particles. Thus, large particles are much more likely to be
collected by passive samplers [Haig et al. 2016; Reponen et al. 2011b]. As a result of these
limiting variables, results from passive bioaerosol sampling cannot be directly related to
the concentration of airborne particles and may not correlate well with results from active
sampling [Reponen et al. 2011b]. However, some authors have proposed that passive
sampling may be useful in evaluating the likelihood that bioaerosol particles will
contaminate surfaces such as open wounds in operating rooms, since they mimic the
contamination event more closely than does an active sampler [Friberg et al. 1999; Haig et
al. 2016; Pasquarella et al. 2000].
Passive bioaerosol collectors are often placed 1.5 to 2 meters above the ground to avoid
collection of large dust particles from sources other than airborne particles, such shoes,
clothing, skin and animals [Frankel et al. 2012; Lioy et al. 2002; Noss et al. 2008; Rintala et
al. 2012]. Grills, screens or shields may also be placed around or over the collection device
to screen out large debris [Brown et al. 1996; Wagner and Macher 2003; Whitehead and
Leith 2008; Wurtz et al. 2005].
Settle plates
Settle plates (also called settling plates or sedimentation plates) are culture plates
containing nutrient agar that are opened and placed collection-side up in a location of
interest. Airborne particles are allowed to settle onto the plates for a specified time,
and the plates are then closed, incubated and inspected for growth. Settle plates are

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-24 of BA-115
Sampling and Characterization of Bioaerosols
commonly used to assess airborne microbial contamination and are listed in methods
and standards from the ISO, the American Public Health Association (APHA) and the
United States Pharmacopeia (USP) [Dyer et al. 2004; ISO 2003; USP 1997]. However,
because the results from settle plates cannot be directly compared to the amount of
airborne microbes, they should only be used for qualitative, not quantitative,
evaluations. The CDC recommends the use of high-volume air samplers rather than
settle plates when investigating airborne fungal spore contamination in health care
facilities [CDC 2003].
Settle plate methods suffer from a lack of standardization of methodology, which
makes results difficult to compare. Pasquarella et al. [2000] reviewed the use of settle
plates and proposed an Index of Microbial Contamination (IMA) to standardize the
use of settling plates. To measure the IMA, 90 mm culture plates are placed 1 meter
above the floor and 1 meter from any walls, and collect settled particles for 1 hour
(called the 1/1/1 scheme). The number of colony-forming units (CFUs) detected on
each plate is then used to calculate the IMA in CFUs/dm2/hour [Pasquarella et al.
2000].
Electrostatic dust collectors
Noss et al. [2008] developed a method called the electrostatic dustfall collector (EDC)
that collects settling airborne particles onto four electrostatically-charged cloths.
EDC’s have been used in studies of culturable bacteria and fungi, endotoxin, glucan
and inflammatory mediators in airborne particles [Adams et al. 2015; Frankel et al.
2012; Huttunen et al. 2016; Kilburg-Basnyat et al. 2016; Kilburg-Basnyat et al. 2015;
Noss et al. 2010; Noss et al. 2008]. Noss et al. [2008] and Frankel et al. [2012] reported
good correlations between the EDC and active aerosol samplers. Adams et al. [2015]
compared EDC’s to Petri dishes and other passive collection materials and found that
the results correlated reasonably well, but that a rigorous extraction protocol was
required to get consistent results from the EDC’s. Brown et al. [1996] developed a
passive electrostatic-based personal aerosol sampler and reported that it gave a
reasonable correlation with inhalable dust measurements at farms and a rubber plant.
Other passive bioaerosol samplers
The UNC Passive Aerosol Sampler consists of a 6.8 mm diameter collection substrate
mounted on a scanning-electron microscope stub and shielded by a protective screen
[Wagner and Macher 2003; Whitehead and Leith 2008]. Airborne particles settle or
diffuse onto the substrate and can be analyzed by optical or electron microscopy.
Other investigators have used aluminum sheets in boxes, Petri dishes, and sheets of
various plastic materials as passive bioaerosol collectors [Adams et al. 2015; Meadow et
al. 2015; Wurtz et al. 2005].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-25 of BA-115
Sampling and Characterization of Bioaerosols
i. Settled dust collection devices
The collection and analysis of dust that has settled onto floors, carpets, and other surfaces
is widely used as a means of identifying bioaerosols in buildings, especially allergens,
endotoxin and molds [Hung et al. 2005; Lioy et al. 2002; Martyny et al. 1999; Morey 2007;
Rintala et al. 2012]. Settled dust sampling allows for the collection of large quantities of
material, provides a long-term sample, and does not require a dedicated sampling device
for each location. Dust assays allow quantitative data to be generated per weight and
surface area of dust. Some investigators find it useful to compare different sites in a
building or to sample before and after remediation efforts to see if the source of a
bioaerosol has been eliminated.
Settled dust will vary within a building depending upon the location and collection surface
[Lioy et al. 2002; Rintala et al. 2012]. In addition to settling from the air, dust can be
produced by a variety of other mechanisms, making it difficult to distinguish the source.
Floor and carpet dust, for example, will include outside material brought in by shoes, skin
flakes, clothing fibers and animal dander. Sampling locations well above floor level are
often chosen to minimize the amount of dust that is not from settled airborne particles
[Frankel et al. 2012; Rintala et al. 2012].
Vacuums
The US Department of Housing and Urban Development has developed a protocol for
the vacuum collection of home dust samples to test for allergens [HUD 2008].
Vacuum collection of settled dust from floors and carpets has been used to determine
the Environmental Relative Moldiness Index (ERMI), which is a measure of mold
contamination in homes [Kettleson et al. 2015; Reponen et al. 2012; Reponen et al.
2011a; Taubel et al. 2016; Vesper et al. 2013; Vesper et al. 2007]. ERMI is discussed in
more detail later in this chapter. Note that vacuuming can increase the levels of
bioaerosols in a location. Thus, air sampling should be completed before collecting
surface samples by vacuuming [Hung et al. 2005; Hunter et al. 1988].
Swabs
Swabs are widely used to collect airborne material that has settled onto surfaces. Swabs
are also used to identify microbial contaminants that may be colonizing building
materials within the indoor environment. However, obtaining consistent and reliable
results from swab sampling is far more difficult than is often appreciated, and careful
attention is needed to the choice of swab material, elution media, and method of
swabbing. If swab samples are to be cultured, aseptic technique is needed to avoid
contamination. ASTM International has a standard for collecting fungal material by
swab [ASTM 2012]. The APHA has published a standard method for swab sampling of

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-26 of BA-115
Sampling and Characterization of Bioaerosols
food-contact surfaces [Dyer et al. 2004], while the USP and ISO have standards that
include swab sampling for microbiological contamination in clean rooms [ISO 2003;
USP 1997].
An example of a validated protocol for swab sampling is that provided by NIOSH for
surface sampling for Bacillus anthracis spores [Hodges et al. 2010; Hodges et al. 2006;
NIOSH 2012b]. In this procedure, a defined area is first outlined using a template or a
ruler and masking tape. A sterile macrofoam swab is then moistened using a buffer
solution that neutralizes disinfectants. The surface is swabbed using horizontal strokes,
followed by vertical strokes, and finally diagonal strokes, and the swab is then placed
in a sterile tube for transport and analysis. Aseptic technique is used throughout the
procedure.
The choice of swab material can have a significant impact on the collection of
microorganisms from a surface. Moore and Griffith [2007] studied the recovery of
Escherichia coli and Staphylococcus aureus from stainless steel squares using nylon-
flocked swabs and spatulas, cotton swabs and rayon swabs. They reported that nylon-
flocked and cotton swabs were equally effective at removing bacteria from dry
surfaces, but that cotton swabs removed bacteria more effectively from wet surfaces
than rayon or nylon-flocked swabs. However, nylon-flocked swabs and spatulas
released the bacteria into the elution media more readily than rayon swabs, which in
turn released more bacteria than cotton swabs. For viruses, polyester-tipped swabs
were found to be more effective than cotton swabs or antistatic wipes at recovering
MS2 bacteriophage from stainless steel and plastic [Julian et al. 2011], while
macrofoam swabs performed best when recovering wet or dried norovirus from
stainless steel surfaces, followed by cotton, rayon and polyester swabs [Park et al.
2015].
The elution media used to wet the swabs and recover the bacteria from the swabs also
can have a substantial effect on sampling. Moore and Griffith [2007] tested eleven
different swab wetting solutions containing various combinations of salts, surfactants
and nutrients. They found that the recovery efficiency varied widely depending upon
the species of bacteria, type of swab, and whether the surface was wet or dry. For MS2
bacteriophage, saline or Ringer’s solution (an isotonic salt solution) worked better
than viral transport media or pure water [Julian et al. 2011]. It is important to note
that the elution media must both remove the biological material from the surface and
subsequently elute it from the swab in order to be effective.
Although they may be overlooked, storage conditions play an important role in swab
sampling. After sample collection, room temperature storage of moist swabs may lead
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-27 of BA-115
Sampling and Characterization of Bioaerosols
to microbial growth if the elution media or swab contain nutrients, while the presence
of chemicals such as Tween 80 may reduce viability over time. These problems can be
alleviated by placing the swabs in cold storage as quickly as possible [Moore and
Griffith 2007].
Wipes
All of the considerations and limitations of swab sampling also apply to wipe
sampling. Swabs are typically more useful for small surfaces and hard-to-reach
locations, while wipes are more effective at collecting dust from large non-porous
surfaces [NIOSH 2012b]. Electrostatic wipes have been used to collect settled dust for
studies of mold and endotoxin [Bolaños-Rosero et al. 2013; Thorne et al. 2005].
However, Thorne et al. [2005] found that wipes and gloves themselves were frequently
contaminated with endotoxin and needed to be tested before use.
Adhesive tape
Adhesive tape can be used to collect dust samples from surfaces for microscopic
examination (this is called tape lift or cellotape sampling) [ASTM 2014c; Martyny et al.
1999; Morey 2007]. Typically, a section of adhesive tape is gently pressed onto a
surface of interest, removed with a slow steady force, and then attached to a glass slide
or placed in a vial. The samples are relatively simple to collect, but the results depend
upon the ability of the examiner to identify microorganisms and their fragments, and
do not provide a quantitative assessment of exposure.
Contact plates
Contact plates are typically round culture plates in which the agar is poured so that the
top of the agar forms a meniscus slightly above the top rim of the plate. A surface
sample is collected by inverting the plate and pressing the agar directly onto a flat
surface of interest. The plate is then removed, incubated and inspected for microbial
growth. This sampling method is often called the replicate organism direct agar
contact (RODAC) procedure, and it is commonly used for biocontamination
monitoring in the pharmaceutical and food industries [Dyer et al. 2004; ISO 2003; USP
1997]. Because many of the surfaces of interest in these industries are routinely
disinfected, contact plates are available with agars that contain neutralizers for
disinfectants. One report indicated that nitrocellulose membranes were slightly more
effective than RODAC plates at surface sampling, and are easier to use on curved
surfaces [Poletti et al. 1999].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-28 of BA-115
Sampling and Characterization of Bioaerosols
j. Heating, ventilation and air conditioning (HVAC) filters
Building HVAC systems filter large quantities of outside and recirculated inside air as they
maintain environmental conditions inside buildings. Researchers have taken advantage of
these existing filtration systems as a way to study bioaerosols in a variety of structures
[Goyal et al. 2011; Haaland and Siegel 2016; Noris et al. 2011]. Testing the collected
particulate material on HVAC filters provides an inexpensive way of studying bioaerosols
collected from large volumes of air over long time periods. However, some limitations
must be kept in mind. Extracting bioaerosols from these filters can be difficult and the
methods require validation [Farnsworth et al. 2006]. Many microorganisms lose viability
after collection, so although PCR-based methods may be effective, culture-based methods
likely will not work except for very hardy microbes [Farnsworth et al. 2006]. Finally,
commonly-used HVAC filters can have relatively low collection efficiencies, especially for
small particles [ASHRAE 2009]. Haaland and Siegel [2016] reviewed 60 studies in which
HVAC filter analyses were used to study bioaerosols in buildings.
k. Real-time bioaerosol monitoring
Many biological molecules have an intrinsic autofluorescence, and this phenomenon has
been used as the basis for continuous real-time bioaerosol detection systems [Pöhlker et al.
2012]. This technique is most commonly employed for studies of atmospheric bioaerosol
particles and for biodefense and biosecurity applications. These systems can distinguish
biological from non-biological particles, and can usually provide information about the
particle size and some characteristics of the bioaerosols. One device, the TSI Ultraviolet
Aerodynamic Particle Sizer (UV-APS), was used in several studies [Bhangar et al. 2016;
Hairston et al. 1997; Kanaani et al. 2008]; it has been replaced by an updated version called
the Fluorescence Aerosol Particle Sensor (FLAPS) III. Other real-time bioaerosol detectors
include the BioScout [Saari et al. 2014], the Wideband Integrated Bioaerosol Sensor
(WIBS-4) [Toprak and Schnaiter 2013], and the Fido B2 (formerly called the
Instantaneous Bioaerosol Analysis and Collection, IBAC) [Santarpia et al. 2013].
4 Considerations for bioaerosol sampling
a. Development of a bioaerosol sampling strategy
The first step in designing a sampling strategy for bioaerosol sampling is to determine the
purpose of the sampling [ASTM 2014a]. For example, bioaerosol sampling may be
conducted to estimate worker exposure to bioaerosols, or to select or evaluate engineering
controls to reduce exposures, or to identify the source of a bioaerosol. A sampling strategy
then should begin with an overview of the site of interest and development of initial
hypotheses regarding the types, sources and distributions of bioaerosols. After this, the
sampling methods, times, durations, and the analytical methods can be selected. Note that

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-29 of BA-115
Sampling and Characterization of Bioaerosols
bioaerosol sampling is almost always done in conjunction with the collection of other
types of data, such as worker health information, visual observations, air flow
measurements, surface sampling, and information about possible sources.
b. Sampling locations
The sampling locations should be selected to assist in evaluation of the working
hypotheses about possible exposures [ASTM 2014a]. If worker exposures are being
evaluated, then the samplers should be placed in areas occupied by the workers. If
contamination of a ventilation system is being examined, then sampling in the system and
at the ventilation louvers would be appropriate. Care must be exercised to ensure that
people do not tamper with the samplers and that microorganisms on surfaces or in duct
work are not inadvertently aerosolized.
Bioaerosol samples should be drawn directly into the sampler rather than being
transported to the sampler by tubing. If transport tubing must be used, it should be as
short and straight as possible. Abrupt flow constrictions and bends in the tubing should be
especially avoided, as considerable sample deposition can occur at these locations. The
tubing diameter should be large enough that the flow is not turbulent and that the d50 of
any bends is well above the size of the bioaerosol particles [Pui et al. 1987; Tsai and Pui
1990]. The tubing should be made of a material that does not lead to losses through
electrostatic deposition [Liu et al. 1985]. A review of the many issues surrounding the
transporting of aerosols through sampling lines is provided by Brockmann [2011].
Personal aerosol sampling provides a much better representation of worker and resident
exposure to aerosol particles than area (static) sampling [Cherrie et al. 2011; Kissell and
Sacks 2002; Rodes and Thornburg 2005]. However, most samplers for viable bioaerosols
do not lend themselves to personal sampling. Thus, a combination of personal and area
sampling may be necessary to fully characterize the exposure [Toivola et al. 2002].
c. Concentrations of indoor and outdoor bioaerosols
Indoor bioaerosol sampling is conducted in occupational (industrial, education, and office
environments) and non-occupational (residential and buildings) settings. Outdoor
bioaerosol sampling is often performed to provide comparative data for indoor sampling
and to help determine possible sources of contaminants. Outdoor bioaerosol sampling also
is conducted in occupational environments such as agricultural settings, composting sites
and sewage treatment plants [Environment Agency 2009; Lee and Liao 2014; Masclaux et
al. 2014]. In addition, outdoor sampling may be performed for pollen and fungi to assist
allergists in their treatment of patients by identifying taxa distribution and concentrations
in air over time.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-30 of BA-115
Sampling and Characterization of Bioaerosols
The concentrations of bioaerosol particles vary widely depending upon the meteorological
parameters, the location of sources, the time of year and the amount of ventilation.
Shelton et al. [2002] studied 1,717 buildings in the United States. They found that outdoor
levels of airborne fungi are usually higher than indoor levels, and that fungal levels were
highest in the fall and summer and lowest in the winter and spring. Outdoor levels varied
from 1 to more than 8,200 colony-forming units (CFU)/m
3
of air, with a median of 540
CFU/m
3
. Indoor levels ranged from 1 to over 10,000 CFU/m
3
, with a median of 82
CFU/m
3
. An examination of fungi in flood-damaged homes found fungal concentrations
of 1,100 to 8,400 spores/m
3
outside and 500 to 101,100 spores/m
3
inside [Reponen et al.
2007]. An investigation of 100 large office buildings by Tsai and Macher [2005] found that
airborne bacterial concentrations tend to be higher outdoors than indoor (except for
Gram-positive cocci). Outdoor concentrations tended to be higher in the winter (194 vs.
165 CFU/m
3
), while indoor concentrations were higher in the summer (116 vs. 87
CFU/m
3
). Forty-one percent of the bioaerosol samples were below the detection limit, and
>95% of the culturable bacteria were mesophilic (grow at moderate temperatures). In a
report on agricultural workers working in animal confinements, Lee et al. [2006] found
breathing zone culturable bioaerosol exposures of 300 to 36,000 CFU/m
3
for fungi, 3000 to
3.3 x 108 CFU/m
3
for bacteria, and up to 2,800 CFU/m
3
for actinomycetes. During grain
harvesting, workers were exposed to culturable bioaerosol levels of 82,000 to 7.4 × 106
CFU/m
3
for fungal spores, 40,000 to 1.4 × 106 CFU/m
3
for bacteria, and up to 2.6 × 104
CFU/m
3
for actinomycetes.
If one or more genera of fungi or bacteria are found indoors in concentrations greater than
outdoor concentrations, then the source of amplification may need to be found and
remediated. When conducting indoor bioaerosol sampling, it is advisable to sample
before, during, and after the sampling area is occupied, including times when the heating,
ventilating, and air conditioning system is activated and inactivated.
d. Viable and nonviable bioaerosols
Viable microorganisms are metabolically active (living) organisms with the potential to
reproduce, grow and colonize. Viruses are not metabolically active but are considered
viable if they are capable of reproducing in an appropriate cellular host. Viable
microorganisms may be culturable or non-culturable. Culturable organisms reproduce
under controlled laboratory conditions. Non-culturable organisms do not reproduce in
the laboratory because of intracellular stress or because the conditions (e.g., culture
medium or incubation temperature) are not conducive to growth. Some bacteria can be
very difficult or impossible to culture from bioaerosols. For example, although human
Mycobacterium tuberculosis is readily transmitted among people and from people to
Guinea pigs, it has never been successfully cultured from an environmental aerosol

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-31 of BA-115
Sampling and Characterization of Bioaerosols
sample, probably because of its extremely low airborne concentrations and slow growth
rate [Nardell 2016]. Other bioaerosols such as Histoplasma capsulatum or Pneumocystis
carinii may take weeks to grow or may not even grow in culture at all [Dennis 1990; Ibach
et al. 1954]. As the name implies, viable bioaerosol sampling involves collecting a
bioaerosol and culturing the collected particles. Only culturable microorganisms are
enumerated and identified, thus leading to an underestimation of bioaerosol
concentration. Non-viable and viable but non-culturable microorganisms are often
studied by collecting them with a dry aerosol sampler or a membrane filter. The
microorganisms are then enumerated and identified using microscopy, classical
microbiology, molecular biology, or immunochemical techniques [Hung et al. 2005;
Macher 1999; Reponen et al. 2011b; Tortora et al. 2013].
Assessment of viable bacteria is also dependent on a number of variables including
nutrient media, temperature and culture conditions. In indoor environments the
collection of viable bacteria may be confounded by endogenous bacterial microflora such
as Staphylococcus epidermis that sheds with skin flakes [Hung et al. 2005]. Concentrations
of viable bacteria have been reported to be as high as 105 CFU/m
3
in indoor environments;
however, like fungi, the proportion of the total bacterial burden may be higher if non-
viable bacteria are also included [Hung et al. 2005]. In addition, viable assessment of
several bacterial species of clinical significance may not be the best approach as these
bacteria do not remain viable in the air. Alternative methods such as immunoassays or
molecular-based methods may provide suitable approaches for quantifying bacterial
pathogens.
e. Bioaerosol particle sizes
As noted earlier, the aerodynamic diameter (d
ae
) of an airborne particle is the most
important factor determining how long it will remain in the air, how likely it is to be
inhaled, and where it will deposit in the respiratory tract. The sizes of bioaerosol particles
can range from tens of nanometers for small fragments to hundreds of micrometers for
pollen, fungi or large agglomerations. However, most of the bioaerosol particles of interest
in the indoor environment fall between about 100 nm and 10 µm [Nazaroff 2016]. For
bacteria, vegetative cells typically have physical diameters of about 0.2 to 2 µm and are 2 to
8 µm in length, while bacterial spores are somewhat smaller [Tortora et al. 2013]. Airborne
particles containing bacteria were found to have aerodynamic diameters of about 1 to 3
µm in indoor environments [Gorny et al. 1999; Kujundzic et al. 2006; Meklin et al. 2002].
Mycobacterium tuberculosis is a rod-shaped bacteria with a length of about 6.6 µm [Schafer
et al. 1999]. When aerosolized from a liquid culture, M. tuberculosis DNA was found in
particles with aerodynamic diameters of 0.6 to 1.8 µm [Schafer et al. 1999]. Aerosolized
Mycobacterium bovis BCG (a commonly-used surrogate for M. tuberculosis) was found in

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-32 of BA-115
Sampling and Characterization of Bioaerosols
particles with aerodynamic diameters of 0.5 to 9.9 µm [Schafer et al. 1998]. Air sampling
around indoor whirlpools in a public facility found airborne mycobacteria DNA in
particles with aerodynamic diameters of 0.5 to 9.9 µm [Schafer et al. 2003]. Actinomycete
spores tend to be smaller, with aerodynamic diameters of cultured spores ranging from 0.6
to 1.5 µm [Madelin and Johnson 1992; Reponen et al. 1998]. Fungal spores have physical
diameters of about 0.5 to 30 µm or larger, while the aerodynamic diameters of airborne
fungal spores and spore clusters are reported to be from 0.9 to 5 µm [Eduard 2009;
Hussein et al. 2013; Reponen et al. 2011b].
Airborne microorganisms are often present as parts of aggregations, droplets or
agglomerations that can be much larger than the size of the native microorganism. In
indoor environments with large amounts of other aerosol particles like cigarette smoke,
bacteria have been found on particles with aerodynamic diameters up to 10 µm, which was
larger than airborne bacterial particles in cleaner environments. This was thought to occur
because the aerosol particles were forming agglomerates [Gorny et al. 1999]. In a farm
study, airborne Actinomycetes and fungal spores were more likely to be found in
aggregates in environments with higher spore concentrations [Karlsson and Malmberg
1989]. In two studies of airborne influenza virus in health care facilities, about half of the
airborne virus was found in particles with aerodynamic diameters of 4 µm or greater, even
though the virus itself is only about 100 nm in diameter, because the virus was contained
in aerosolized droplets of respiratory fluids [Blachere et al. 2009; Lindsley et al. 2010a].
Agglomerates of fungal spores can break apart upon impaction inside an impactor and be
collected on subsequent stages with smaller cut-off diameters [Trunov et al. 2001].
Bioaerosols may also be present as cellular fragments that are much smaller than the
source microorganisms. Endotoxins are fragments of the cellular walls of Gram-negative
bacteria that have been implicated in a variety of illnesses [Eduard et al. 2012; Jacobs 1989;
Olenchock 2002]. Fragments of fungal cell walls also are thought to be associated with
several types of adverse respiratory health effects [Green et al. 2011; Green et al. 2006b;
Olenchock 2002]. Very high levels of fungal fragments have been measured in flood-
damaged homes contaminated with mold [Reponen et al. 2007]. Fungal fragments also
contain a variety of secondary metabolites, mycotoxins, beta-glucan, antigens and
allergens [Green et al. 2011; Green et al. 2006b]. In one study of indoor air in homes, the
majority of the endotoxin and fungal wall material was found in particles with
aerodynamic diameters of less than 1 µm [Adhikari et al. 2013]. Another study found
considerable amounts of endotoxin in aerosol particles from metalworking fluids that
were between 0.16 and 0.39 µm [Wang et al. 2007]. Indoor and outdoor measurements of
endotoxin levels found that the largest proportion was detected in particles with
aerodynamic diameters of less than 1 µm [Kujundzic et al. 2006].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-33 of BA-115
Sampling and Characterization of Bioaerosols
It is common to use an aerosol spectrometer in conjunction with bioaerosol sampling to
better understand the size distribution of the airborne particles. One consideration when
interpreting the data is, of course, that the large majority of these devices do not
distinguish between biological and non-biological aerosols. Another less-obvious factor is
that while a few aerosol spectrometers such as the TSI Aerodynamic Particle Sizer measure
the aerodynamic diameter of the airborne particles, many aerosol spectrometers measure
particles using light scattering and thus provide an approximate physical diameter instead
[Hinds 1999; Sorensen et al. 2011]. The difference between the aerodynamic and optical
diameters may be significant depending upon the shape and density of the particles.
f. Temperature and humidity
The temperature and humidity of the environment can affect the size of bioaerosol
particles, the viability of airborne microorganisms, the growth of microorganisms on
surfaces, and the amount of electrostatic charges on aerosols and surfaces. Because of these
effects, the environmental temperature and humidity should be recorded during
bioaerosol sampling.
Water evaporates rapidly from wet aerosol particles [Hinds 1999]. If an airborne particle is
initially an aqueous solution containing non-volatile substances such as salts and organic
material, and if the relative humidity is above the crystallization relative humidity (CRH,
also called the efflorescence relative humidity), then some of the water will evaporate and
the solution will become more concentrated, but the particle will remain liquid. If the
relative humidity is below the CRH, then all of the water will evaporate (that is, the particle
will desiccate) [Nicas et al. 2005]. Similarly, if an airborne particle is initially a dry
combination of salts and organic material, and if the relative humidity is below the
deliquescence relative humidity (DRH), then the particle will remain desiccated. However,
if the relative humidity is above the DRH, then the particle will absorb water until it
liquefies and becomes an aqueous solution. The DRH is always greater than the CRH
[Nicas et al. 2005]. A particle in an environment above its CRH (or DRH if it was initially
dry) will be larger and heavier and will settle faster than the same particle when the
humidity is below the CRH, which can affect the size and amount of bioaerosol particles
that are collected during sampling [Mikhailov et al. 2004]. This phenomenon was seen in a
study of particles in human exhaled breath, where the particles detected in low humidity
air were substantially smaller than those detected when the air was more humid
[Holmgren et al. 2011].
Bioaerosol particles may also undergo an increase in size when the humidity increases due
to water absorption and swelling of hygroscopic components. An increase in relative
humidity has been shown to increase the aerodynamic diameter of fungal spores [Madelin

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-34 of BA-115
Sampling and Characterization of Bioaerosols
and Johnson 1992; Reponen et al. 1996]. Similar results have been reported for
Actinomycetes spores [Madelin and Johnson 1992].
For airborne viruses, survival decreases as air temperature increases [Ijaz et al. 2016; Tang
2009]. Exposing most viruses to temperatures of 60°C or higher for 60 minutes will
inactivate them, although the viruses can be somewhat protected if they are encased in
organic material [Tang 2009]. For example, in one set of experiments, airborne particles
containing vaccinia virus, influenza virus, and Venezuelan equine encephalomyelitis virus
all showed higher survival rates at 7-12°C than at 21-24°C, and still lower survival at 32-
34°C [Harper 1961]. Aerosol transmission of influenza virus among Guinea pigs is
blocked at air temperatures of 30°C [Lowen et al. 2008]. The effect of humidity on virus
survival depends upon the virus; in general, viruses with lipid envelopes tend to survive
better at low humidity, while non-enveloped viruses survive better at high humidity [Ijaz
et al. 2016; Tang 2009]. For example, influenza viruses and coronaviruses have enveloped
capsids, and both survive better at low humidities compared to high [Ijaz et al. 1985a; Ijaz
et al. 2016; Noti et al. 2013; Schaffer et al. 1976]. On the other hand, rotaviruses and
rhinoviruses have non-enveloped capsids and survive better at high humidities compared
to low [Ijaz et al. 1985b; Ijaz et al. 2016; Karim et al. 1985].
The survival of airborne bacteria also decreases as air temperature increases; the survival of
virtually all airborne bacteria declines when temperatures are above 24°C [Ijaz et al. 2016;
Tang 2009]. However, as with viruses, the effects of humidity on bacterial survival are
much more complex, and depend not only upon species but also upon the methods of
culture and aerosolization [Cox 1989; Tang 2009]. In field experiments in a greenhouse,
survival of certain bacteria was 35- to 65-fold higher at 80% RH than at 40% [Walter et al.
1990]. In laboratory experiments, survival of certain bacteria was virtually complete at low
RH but was reduced at RH values above 80% [Cox 1968]. Higher humidities can also
significantly decrease the efficacy of ultraviolet germicidal irradiation (UVGI) for reducing
levels of viable airborne bacteria [Peccia et al. 2001]. Cox [1987] believes the potential for
the movement of the solvent water is an important environmental criterion in assessing
survivability of bacteria, viruses, and phages.
Fungi and fungal spores generally are better able to withstand environmental stresses
compared to vegetative bacteria and viruses [Ijaz et al. 2016; Tang 2009]. Warm
temperatures, wet substrates and humid air conditions favor the growth of fungi on
surfaces [Eduard 2009; Tang et al. 2015]. Temperature can induce morphological changes
in dimorphic fungi such as the pathogen Histoplasma capsulatum [Salvin 1949]. It is not
clear, however, how air temperature and humidity affect the viability of airborne fungi and
fungal spores [Tang 2009].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-35 of BA-115
Sampling and Characterization of Bioaerosols
g. Electrostatic effects
Aerosol particles in the workplace can be highly charged, and the electrostatic charge can
vary considerably depending upon the aerosol generation mechanism and the particle
characteristics [Johnston et al. 1985]. Aerosol particles are especially prone to develop
electrostatic charges in low humidity environments [Baron and Deye 1990]. Like most
particles, freshly generated microbial aerosols are nearly always electrostatically charged
unless steps are taken to neutralize them. Lee et al. [2004b] found that airborne fungi and
bacteria carried a net negative charge in most of the laboratory and field environments
that they studied. Mainelis et al. [2002] found that a strong positive electrostatic charge
reduced the viability of Pseudomonas fluorescens bacteria but did not affect Bacillus subtilis
spores.
The effect of electrostatic charge on aerosol collection is often overlooked, resulting in the
possible bias of sampling results [NIOSH 2016a; Vincent 2007]. Aerosol samplers made of
non-conductive plastics can develop substantial electrostatic charges, which can degrade
their performance significantly [NIOSH 2016a; Baron and Deye 1990]. The use of
polyethylene or polytetrafluoroethylene (PTFE) tubing to transport air streams to a
sampler can remove a sizeable amount of aerosol particles by electrostatic deposition [Liu
et al. 1985]. As noted above, the use of plastic Petri dishes in an Andersen impactor can
result in bioaerosol particle losses [Andersen 1958; Kuo 2015]. Whenever possible, it is
better to use aerosol samplers made of conductive materials such as metals or specially-
treated plastics [NIOSH 2016a].
h. Flow calibration
Accurate airflow rates are very important in calculating the concentration of
microorganisms in the air. All samplers should be calibrated before and after sampling to
ensure that the flow rate is within the manufacturer's specifications and does not change
from the initial calibration. Calibration may be performed using a primary standard such
as a spirometer or bubble calibrator. Where it is not possible to calibrate using a primary
standard, a calibrated secondary standard such as a dry gas meter may be used. The
calibration of such a secondary standard should be traceable to a primary standard. A
detailed explanation of the calibration of airflow rates is given by McCammon Jr. and
Woebkenberg [NIOSH 2016c].
i. Blanks
Laboratory media blanks are unexposed, fresh samples of media, such as agar plates, filters
and impinger fluids. These samples are generally not taken into the field. Before using any
batch of media, incubate at least three culture plates under the same conditions as planned

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-36 of BA-115
Sampling and Characterization of Bioaerosols
for the field samples, in order to check for sterility of the media. Approximately five media
blanks should be included with each sample set. If the samples are to be analyzed by an
outside laboratory, consult the specific laboratory procedure for the number of blanks to
be submitted. Similarly, blank filters should be processed in the same manner as planned
for field samples in order to check for contamination.
Field blanks are simply unopened, fresh media samples that are handled in the same way
as field samples, including labeling, except that no air is drawn through the sampler. The
generally recommended practice for the number of field blanks is to provide at least two
field blanks for every 10 samples with a maximum of 10 field blanks for each sample set.
5 Selection of bioaerosol samplers
The first step in selecting a bioaerosol sampling device is to establish the purpose of the
sampling. Once the goal of the bioaerosol sampling is determined, the appropriate sampling
methods may be chosen. The selected bioaerosol sampler must be capable of high efficiency
particle collection within the physical and biological conditions required by the
microorganisms to be sampled. The most appropriate sampling methods will be dictated in
part by the techniques that will be used to analyze the sample. Methods for bioaerosol sample
analysis are discussed in the next section. A list of some manufacturers and suppliers of
bioaerosol sampling equipment and supplies is shown in Appendix I. The characteristics of
several commonly used bioaerosol samplers are shown in Appendix II.
a. Sampling for airborne bacteria and fungi
Choosing a bioaerosol sampler for bacteria and fungi begins by deciding how the
bioaerosol will be analyzed, and in particular whether the viability of the bacteria or fungi
will be evaluated. Culturable bioaerosol sampling instruments must minimize injury
during the collection process and maintain the culturability of the collected
microorganisms. If the sample will not be cultured, then the samples usually can be
collected dry using a membrane filter, cyclone, impactor, or a combination of these. Dry
collection is typically simpler and less expensive to perform, and filters and cyclones can
handle a wide range of particle concentrations. Organisms that are difficult or impossible
to grow in culture are often collected using dry techniques and assessed using polymerase
chain reaction (PCR) based methods, which have the advantage of speed and specificity.
PCR has been used for rapid detection of Histoplasma capsulatum and mycobacteria [Reid
and Schafer 1999; Schafer et al. 1999; Schafer et al. 2003]. A DNA-based mold specific
quantitative PCR (msQPCR) method is widely used to evaluate indoor fungal bioaerosols
in the academic, government and commercial sectors, and is the basis for the
Environmental Relative Moldiness Index (ERMI) used to quantify mold contamination in

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-37 of BA-115
Sampling and Characterization of Bioaerosols
homes [Kettleson et al. 2015; Vesper et al. 2013]. The ERMI and other PCR-based assays
are discussed in greater detail later in this chapter.
If viability is to be studied, then the samples usually will need to be collected with an
impinger or in an Andersen impactor loaded with agar plates, because many
microorganisms will lose viability due to damage or desiccation if collected dry [Cox 1987;
Hung et al. 2005]. For example, a membrane filter sampler is not appropriate for sampling
culturable Escherichia coli because the cells desiccate and become either nonviable or
viable but not culturable under these conditions [Jensen et al. 1992]. Similar results have
been reported for other bacteria and fungi [Macher and First 1984; Wang et al. 2001].
Depending upon the target microorganism, impingers may be filled with distilled water or
a buffered isotonic solution, sometimes with antifoaming agents to reduce foaming and
proteins to enhance survival. Mineral oil has also been used in impingers instead of
aqueous solutions to avoid evaporation [Lin et al. 2000]. Impactors are loaded with agar
plates; the choice of agar depends upon the microorganisms of interest and the desired
selectivity (discussed in the next section).
As noted previously, depending upon the investigation that is being conducted, the
particle size distribution of the bioaerosol may be very important in the evaluation of the
data obtained. If particle size information is needed to, for example, determine how much
of the bioaerosol is in the respirable size fraction, then a size-selective sampler should be
used for at least some of the collections if possible. For example, if an SAS-Compact
sampler was the selected sampler for collection of culturable Escherichia coli, an Andersen
6-Stage sampler could be used to determine the particle size distribution at each location
sampled. The expected size of the bioaerosol particles is also an important factor in
choosing a sampler. For example, an impactor with a d50 of 4 µm should not be used to
collect Aspergillus niger spores (dae 1-3 µm) because most spores would remain entrained
in the air and pass through the instrument.
NIOSH Method 0800 discusses sampling for culturable airborne bacteria and fungi with
an Andersen cascade impactor [NIOSH 2003b]. Standard methods for the collection of
airborne fungi by inertial impaction are presented in ASTM Standards D7788 [ASTM
2009; ASTM 2014d]. ASTM Standard D7391 also discusses the aspects related to the
laboratory analysis.
b. Sampling for airborne viruses
Airborne viruses are more difficult to study in bioaerosols than bacteria and fungi for a
variety of reasons [Prussin et al. 2014; Verreault et al. 2008]. Viruses are more difficult to
culture because they are obligatory intracellular parasites that require a host cell for

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-38 of BA-115
Sampling and Characterization of Bioaerosols
reproduction [Tortora et al. 2013]. Bioaerosols of pathogenic viruses have been found in
many settings to be present in low concentrations that can be difficult to detect [Blachere
et al. 2009; Bonifait et al. 2015; Lindsley et al. 2010a; Tseng et al. 2010; Yang et al. 2011].
Viruses also are generally more susceptible to damage during aerosol collection than are
bacteria or fungi, although their sensitivity varies widely with the collection method and
species [Appert et al. 2012; Turgeon et al. 2014; Zuo et al. 2013]. Aerosol sampling
methods for viruses have been reviewed by Verreault et al. [2008].
Bacteriophages are viruses that infect bacteria rather than multicellular organisms. They
are used in laboratory aerosol studies as tracers for aerosol particles and as surrogates for
airborne viruses that infect humans [Fisher et al. 2012; Tseng and Li 2005; Turgeon et al.
2014]. Bacteriophages are not known to be hazardous to humans but are of interest to
industries that rely on bacteria such as cheese manufacturers [Verreault et al. 2011].
Polymerase chain reaction (PCR) based methods are often used to study viral bioaerosols.
PCR has the advantages of being very sensitive and very specific, and considerably easier
to perform than viral cultural assays. For this reason, most recent field studies of airborne
viruses have used PCR as the detection method. Examples include studies of viruses in
healthcare facilities [Blachere et al. 2009; Booth et al. 2005; Lindsley et al. 2010a;
Thompson et al. 2013; Tseng et al. 2010], influenza at poultry and pig farms [Corzo et al.
2013; Jonges et al. 2015], airborne viruses in a sewage treatment plant [Masclaux et al.
2014], and respiratory viruses in human coughs and exhaled breath [Gralton et al. 2013;
Lindsley et al. 2010b; Milton et al. 2013].
PCR has both the advantage and disadvantage of not requiring that the virus be viable in
order to be detected. This eliminates the need to preserve viability during and after
collection and allows the use of dry collection methods such as cyclone samplers, dry
impactors and filters, which are simpler and easier to carry out. On the other hand, this
also means that it is unclear whether the airborne virus is infectious or not, which makes
interpretation of data more difficult. This is a common criticism of PCR-based bioaerosol
studies.
If the virus in a bioaerosol sample is to be cultured, in most cases the sample will need to
be collected into an aqueous media using an impinger or wetted surface aerosol collector.
Fabian et al. [2009] showed that collecting airborne influenza virus in aqueous media
using an SKC BioSampler preserved infectivity much better than dry collection using
filters or an impactor. A less-common method is to collect viable viruses using an
Andersen impactor. Gustin et al. [2011] collected airborne influenza virus using an
Andersen impactor by placing a filter and a thin layer of gelatin on top of the agar in the
culture plates. After collection, the gelatin was removed and melted at 37°C to allow

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-39 of BA-115
Sampling and Characterization of Bioaerosols
subsequent culture of the virus. Note that the collection media must be compatible with
the cell culture system used to host the virus.
6 Sample preparation for culturable bioaerosols
Collecting and culturing viable airborne microorganisms is the most common technique used
by industrial hygienists to assess bioaerosols [Macher 1999]. However, the appropriate sample
preparation method is highly dependent upon the microorganism(s) of interest, sample
source, and down-stream analysis. These sampling approaches are further confounded as
viable bioaerosols have been estimated to account for approximately 1% of the total bioaerosol
load, and non-viable bioaerosols are often overlooked [Hung et al. 2005]. In contrast, non-
culturable bacteria and fungi cannot be grown in conventional lab-based conditions, but their
presence is still important from a health perspective [Green et al. 2011; Mitakakis et al. 2003].
Non-viable bioaerosols can be determined through other detection methodologies such as
microscopy, proteomic, immunological, and molecular analysis methods, and some of these
approaches are discussed in section 9. A list of the common bioaerosols encountered in
indoor and outdoor environments, as well as the fungi that are common contaminants of
indoor building materials, can be found in Flannigan et al. [2011].
a. Sample preparation for bacteria and fungi
Viable bacterial and fungal bioaerosol identification is made through the collection,
deposition, and growth of a viable propagule or intact cell on a selected nutrient agar
medium contained in a sterile petri dish or liquid culture suspension [Macher 1999].
These methods are similar for both fungal and bacterial bioaerosols [Flannigan et al. 2011;
Hung et al. 2005]. Selection of the nutrient media, incubation conditions (time and
temperature), and potential damage to the culturable bioaerosol during sampling are
among several critical variables to review before the collection, growth and proliferation of
a viable propagule [Eduard et al. 2012; Hung et al. 2005; Macher 1999]. These parameters
have been reviewed elsewhere, but should be taken into consideration when planning an
environmental survey [Hung et al. 2005; Macher 1999].
Growth media can be defined as either broad or selective [Macher 1999]. As the term
implies, broad nutrient media supports the growth of a diverse number of
microorganisms. In contrast, a selective growth medium, with appropriate energy sources,
nutrients, and pH, is used to enrich growth of the specific microorganism in question and
inhibit the growth of competitive organisms [Macher 1999]. A variety of broad and
selective nutrient media for bacteria and fungi are available to the industrial hygienist and
can be found in Hung et al. [2005] and Macher [1999].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-40 of BA-115
Sampling and Characterization of Bioaerosols
Following sample collection, liquid or agar cultures are incubated at a suitable temperature
and atmosphere (facultative versus aerobic) for an appropriate time. Fast-growing bacteria
may develop microcolonies in hours, while fungi may take days to develop into a visible
colony and perhaps sporulate. Organisms such as Mycobacterium tuberculosis or the
dimorphic fungal pathogens, Histoplasma capsulatum or Blastomyces dermatitidis may
require weeks of incubation to produce visible colonies [ATS 1990; Babady et al. 2011].
For fungi, plates are typically incubated at room temperature (18°C-25°C) or, if it is a
clinically relevant isolate, at 35°C [ACGIH 1989; Baron and Finegold 1990; Hung et al.
2005; Macher 1999]. In contrast, environmental bacteria are grown between 18°C and
28°C, while thermophilic bacteria are grown between 50°C and 58°C [Hung et al. 2005;
Macher 1999].
After allowing for vegetative growth of all viable propagules on the selected nutrient
medium, the number of colonies is identified, quantified and presented as colony forming
units (CFUs) [Eduard et al. 2012]. Media blanks (laboratory and field) should be processed
using the same methods as samples to control for environmental or laboratory
contaminants. A collection of bioaerosol identification manuals is presented in both the
AIHA and ACGIH manuals [Hung et al. 2005; Macher 1999]. Color micrographs of
common fungal contaminants are also presented in Flannigan et al. [2011]. Along with the
quantification of viable microorganisms, taxonomic data and an interpretation of the
datasets are generally reported [Hung et al. 2005].
The interest in detecting and quantifying fungi has increased following consensus
documents that reported associations between fungi in damp indoor environments and
adverse respiratory health effects [IOM 2004; Mendell et al. 2011; WHO 2009]. Compared
to bacteria, additional variables need to be taken into consideration by the industrial
hygienist when evaluating viable fungal bioaerosols including water activity, colony
competition, and carbohydrate nutrient sources [Hung et al. 2005; Macher 1999]. Broad
viable culture approaches favor species belonging to the phylum Ascomycota, as well as
species that outcompete slower-growing species. Several different types of media and
physiological conditions (e.g. temperature) may also need to be employed to assess
complete fungal diversity using this approach. For fungi, selection of the nutrient media
may potentially bias the growth of specific viable fungal bioaerosols. Common nutrient
media include malt extract agar (MEA) supplemented with chloramphenicol or rose
bengal agar to suppress bacterial growth [Hung et al. 2005; Macher 1999]. Cellulose agar
can also be used for the selection of indoor fungal contaminants such as Stachybotrys
chartarum [Hung et al. 2005]. Dichloran glycerol (DG18) can be used to select for those
fungi that are xerotolerant [Flannigan et al. 2011; Hocking and Pitt 1980; Macher 1999].
Temperature and incubation time can also be used to select for specific fungal bioaerosols
such as Aspergillus fumigatus which are capable of growth within human hosts [Flannigan

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-41 of BA-115
Sampling and Characterization of Bioaerosols
et al. 2011; Hung et al. 2005]. A selection of nutrient media and growth conditions that can
be used for viable fungal culture can be found in Hung et al. [2005], Flannigan et al. [2011]
and Macher [1999].
b. Sample preparation for viruses
Because viruses are obligate intracellular parasites, special precautions must be taken in an
effort to minimize damage to the collected virus-laden aerosol. Environmental factors
such as humidity, temperature and gas composition of the air can significantly impact the
infectiousness of a virion and should be monitored closely [Ijaz et al. 2016]. Several studies
have shown that the inactivation of an airborne virus is directly related to the relative
humidity and temperature [Weber and Stilianakis 2008]. In one study, high humidity
levels caused a loss of infectious influenza virus from simulated coughs [Noti et al. 2013].
Similarly, using a ferret animal model, Lowen et al. [2007] demonstrated a correlation
between airborne transmission of influenza and the relative humidity and temperature.
Through the use of an ozone-oxygen delivery system, researchers were able to show that
ozone-mediated reactive oxygen species (ROS) caused lipid peroxidation and subsequent
damage to the lipid envelope and viral capsid [Murray et al. 2008]. Such studies highlight
the importance of collecting viral aerosols under optimal environmental conditions and,
when possible, minimizing the detrimental effects of environmental factors on collected
samples.
Air sampling techniques also may cause damage to the virus and compromise analysis.
Before collecting viral aerosols, the hardiness of the target virus must be taken into
account. Currently, there are over 200 known respiratory viruses that fall under one family
of DNA viruses (Adenoviridae) and four families of RNA viruses (Orthomyxoviridae,
Paramyxoviridae, Picornaviridae and Coronaviridae) [Abed and Boivin 2006]. While all
viruses package their genome in a protective protein coat known as the capsid, some
viruses also possess a lipid bilayer envelope that, as the name implies, surrounds the viral
capsid. Once outside the host, the viral envelope is highly sensitive to desiccation,
temperature fluctuations and readily undergoes degradation. Variations in temperature
can greatly affect viral enzymatic activity and nucleic acid stability [Tang 2009]. As noted
earlier, viruses with lipid envelopes tend to survive better at low humidity, while non-
enveloped viruses survive better at high humidity [Tang 2009]. Also, RNA viruses are
inherently more unstable than DNA viruses due to the presence of the 2’-hydroxyl group
on the ribose sugar molecule of RNA that is susceptible to base-catalyzed hydrolysis and
degradation. Therefore it is critical that collection methods do not disrupt the lipid
membrane and/or compromise the integrity of nucleic acids. Impairment of either the
lipid envelope or nucleic acids can significantly impact detection of the viral aerosol and
lead to false negatives.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-42 of BA-115
Sampling and Characterization of Bioaerosols
To optimize collection efficiency while maintaining infectiousness of the viral aerosol,
researchers must be discriminating when deciding on what type of aerosol sampler to use
and how long the sampling collection period should be. As discussed in Section 3, some
commercially available bioaerosol sampling devices exist, each of which possesses unique
collection properties. In a review by Verreault et al. [2008], liquid impingers were found to
be the most effective sampling devices for capturing small viral particles while maintaining
virion integrity and infectiousness [Verreault et al. 2008].
While liquid impingement preserves and maintains viral integrity (in comparison to dry
impaction), the type of collection medium must also be considered. With culture-based
identification methods, it is of utmost importance to maintain the stability of the collected
viruses while using cell-culture compatible media. The type of liquid medium, as well as
the volume used, are important variables to consider. Virus collection and transport media
are typically isotonic solutions with a buffer to control the pH, protein to protect the virus,
and antibiotics to prevent microbial growth. If used in an impinger, the viral collection
media may also include an antifoaming agent. Specimen handling, storage time and
storage temperature can significantly impact the integrity of sample analysis. Collection
and storage under suboptimal conditions can result in viral inactivation and degradation
of nucleic acids. Bioaerosol samples containing viruses should be processed as soon as
possible after collection. They should be refrigerated or frozen and transported as quickly
as possible. The stability and retention of viability depend upon the virus [Johnson 1990].
7 Identification of culturable bioaerosols
Identification of the microbial taxa is a critical element in the determination of the viable
bioaerosol load in an industrial or occupational environment. The science of classification,
especially the classification of living forms, is called taxonomy. The objective of taxonomy is
to classify living organisms to establish the relationship between one group of organisms and
another, and to differentiate between them based on phenotypic and genotypic characteristics.
The identification of viable fungal bioaerosols has been challenging due to the confusion of
current nomenclature [Flannigan et al. 2011]. As a result, investigators often use synonymous
names that over time have been placed in another group. Familiarity with taxonomy and
nomenclature is critical when undertaking assessments of the viable microbial burden in an
environment [Flannigan et al. 2011]. Several criteria and methods for the classification of
culturable microorganisms are briefly discussed in the following subsections. Besides using
these methods, the nonviable and non-culturable methods of identification discussed in
Section 9 may also be used in combination with these viable methods.
Classical microbiology includes general methods for classifying or identifying
microorganisms. The least specific of these is the observation of growth characteristics.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-43 of BA-115
Sampling and Characterization of Bioaerosols
Growth characteristics include the appearance of the microorganisms in a liquid medium,
colony morphology on solid medium, pigmentation, and arrangement of reproductive
structures such as fungal sexual or asexual spores.
a. Bacteria
Bacteria are prokaryotes with distinguishing morphological characteristics that include the
cell shape, cell size, arrangement of cells, and the presence or absence of flagella, capsules,
or endospores. Simple and differential staining may be performed on bacteria to enhance
visualization and to aid in grouping and identification [Tortora et al. 2013]. In simple
staining, a single basic dye is used that highlights the cellular morphology. Stains such as
methylene blue, carbolfuchsin, crystal violet, or safranin may be used for bacteria.
A differential stain distinguishes among structures or microorganisms based on varying
reactions to the staining procedure. Two examples of differential stains are the Gram stain
and the acid-fast stain. In Gram staining, bacteria are stained and then washed with
alcohol. Gram-positive bacteria possess a cell wall composed of a relatively thick
peptidoglycan layer and teichoic acids, which retains the dye complex. Gram-negative
bacteria possess a cell wall composed of a thin peptidoglycan layer and an outer membrane
which consists of lipoproteins, lipopolysaccharides, and phospholipids, and do not retain
the dye complex when washed [Tortora et al. 2013]. A few of the commercially available
identification kits require a Gram-stain prescreening to assure that the correct reagents are
used. Acid-fast stains are used for some species of bacteria, particularly those of the genus
Mycobacterium, which do not stain readily. In the acid-fast staining process, the
application of heat facilitates the staining of the microorganism [Tortora et al. 2013].
b. Legionella
Bacteria that are placed in the genus Legionella, are the etiological agents of pulmonary
infections called Legionellosis [Fields 2002]. Legionella pneumophila (Figure 1B) is the
most widely known species that has been implicated in Legionnaires’ disease, which can
result in pneumonia. Milder illness with fever and body aches is referred to as Pontiac
fever. Bacteria placed in this genus consist of Gram negative rods and are associated with
freshwater in the environment [ASTM 2015; Macher 1999]. Exposure to warm
temperatures (25-42°C) can result in the growth and proliferation of the bacteria, a
problem that has emerged in water and air handling systems within the indoor built
environment [Hung et al. 2005]. Growth and persistence within Protista have also been
reported [Hung et al. 2005]. For health care facilities, ASHRAE Guideline 12–2000
recommends storing and distributing cold water at <20°C (68°F), whereas hot water
should be stored at >60°C (140°F) and circulated with a minimum return temperature of
51°C (124°F). In other settings, hot water should be stored at ≥40°C (≥120°F) [ASHRAE

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-44 of BA-115
Sampling and Characterization of Bioaerosols
2000]. Building air conditioning cooling towers, humidifiers, or structures used for
bathing such as hot tubs are particularly susceptible to Legionella amplification. Legionella
can be aerosolized within water droplets via abiotic disturbance mechanisms and
disseminated into the breathing zone of the subject. Airborne levels lower than 10
CFU/mL have been associated with Legionnaires’ disease [ASTM 2015; Demirjian et al.
2015; Hung et al. 2005].
Since the identification of L. pneumophila and association with Legionnaires’ disease in
1976, there have been many studies that have focused on a variety of approaches to detect
and mitigate this bacterial species from the built environment. These approaches are
broadly reviewed in Hung et al. [2005]. Along with industrial hygiene practices and
building maintenance, environmental monitoring and surveillance programs are critical to
ensure effectiveness of employed engineering controls and maintenance/disinfection
programs [ASTM 2015]. ASTM International has published a standard for inspecting
water systems and investigating outbreaks [ASTM 2015]. The CDC has also published a
sampling procedure [CDC 2015].
In 2015, ASHRAE published a consensus standard for the primary prevention of
Legionnaires’ disease in building water systems [ASHRAE 2015]. Similar environmental
assessment methods are utilized in maintenance programs and outbreak cases. In this
approach, bulk water samples are typically collected (250 mL to 1 L for non-potable and
1000 mL for potable water), concentrated via filtration, resuspended, and then plated on a
growth medium (such as buffered charcoal yeast extract media) to enable the propagation
and identification of Legionella spp. [CDC 2015]. Samples may also be direct plated, acid
treated, or heat treated to enhance the recovery of the bacterium. Colonies represent the
viable fraction of Legionella in a water sample and these colonies are quantified and
reported as CFU/mL
In addition to viable culture-based approaches, molecular-based methods such as PCR as
well as antibody-based methods have been developed to enable the detection of Legionella.
Air sampling is not considered a reliable method for Legionella surveillance in the built
environment [Hung et al. 2005].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-45 of BA-115
Sampling and Characterization of Bioaerosols
Using an environmental microbiology laboratory with expertise in propagating Legionella
spp. is important when evaluating Legionella contamination of water systems within the
built environment. A number of laboratories participate in the CDC’s Environmental
Legionella Isolation Techniques Evaluation (ELITE) Program. Participation in the
program is voluntary and enables laboratories to test their proficiency in Legionella
isolation and identification techniques against standardized samples. A list of ELITE
member laboratories can be accessed at
https://wwwn.cdc.gov/elite/Public/MemberList.aspx.
c. Fungi
In general, culturable fungi are classified by colony features including the septation of
hyphae and colony morphological phenotypes, including pigmentation and the
presentation of asexual and sexual spores on hyphae. Stains such as Calberla’s solution,
lactophenol cotton blue, periodic acid-Schiff stain, Grocott’s methenamine silver stain,
and calcofluor white may be used in combination with potassium hydroxide (10% KOH)
to resolve these colony structures using microscopic-based approaches [Hung et al. 2005].
The identification and classification of fungal colonies should be performed by an
examiner that is skilled in microbiology and mycology. A number of commercial labs that
employ examiners skilled in the identification of microorganisms encountered in indoor
and occupational environments are accredited by the AIHA Environmental Microbiology
Laboratory Accreditation Program (EMLAP).
In addition to viable culture-based approaches, biochemical, physiological, and nutritional
tests for bacteria and fungi can be used [Flannigan et al. 2011]. These testing strategies
offer identification based on numerous variables including cell wall constituents, pigment
biochemicals, storage inclusions, antigens, optimum temperature and temperature range,
the effect of oxygen on growth, pH tolerance, osmotic tolerance, salt requirement and
tolerance, antibiotic sensitivity, energy sources, carbon sources, nitrogen sources,
fermentation products, and modes of metabolism (autotrophic, heterotrophic,
fermentative, respiratory). As a rule, batteries of such tests, rather than any one individual
test, are used to identify or classify microorganisms. A few commercially available test
batteries are discussed briefly in the section on biochemical approaches.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-46 of BA-115
Sampling and Characterization of Bioaerosols
8 Enumeration of culturable bioaerosols
a. Enumeration of bacteria and fungi
The total concentration of culturable airborne microorganisms in a sample is determined
by collecting the bioaerosol sample on a culture plate or plates (or inoculating culture
plates with a bioaerosol sample), incubating the plates, and dividing the number of
colonies observed on the culture plates by the volume of air sampled. Note that, as
discussed in section 2, the number of colonies counted on an agar plate from a bioaerosol
impactor must be adjusted using a positive-hole correction factor to correct for multiple
microorganisms depositing beneath an impactor hole [Andersen 1958; Leopold 1988;
Macher 1989]. A colony is defined as a macroscopically visible growth of microorganisms
on a solid nutrient medium. Concentrations of culturable bioaerosols collected during air
sampling are normally reported as colony forming units (CFU) per unit volume of air
[Eduard et al. 2012]. CFUs also can be determined from samples collected in a swab or
dust sample collected from the floor or area of contamination [Hung et al. 2005].
Often, it is difficult to identify multiple colonies at one location on a plate because of the
lack of differential colony morphology [Burge et al. 1977]. In addition, some organisms
produce large, spreading colonies while others produce microcolonies. Analysis of plates
containing multiple types of microorganisms can be difficult because the chemicals
secreted by one microorganism might inhibit the growth of other microorganisms at that
same location [Burge et al. 1977]. The morphology of the colony of one microorganism
also may completely obscure that of another, and a fast-grower might obscure a slow-
grower.
b. Enumeration of viruses
Before the advent and mainstreaming of molecular-based detection methodologies, cell
culture-based methods and serological assays were considered the gold standard for the
detection of viral pathogens. Typically, through the use of commercially available
immortal cell lines, researchers can screen collected bioaerosols by inoculating cells and
looking for common cytopathic effects (CPEs) such as rounding of infected cells, fusion
with adjacent cells and lysis of cells. Examples of well-known cell lines that are routinely
used in viral diagnostics include primary rhesus monkey kidney (RhMK) cells, primary
rabbit kidney cells, human lung fibroblasts (MRC-5), human epidermoid carcinoma cells
(HEp-2), human lung carcinoma cells (A549) and Madin Darby kidney cells (MDCK)
[Leland and Ginocchio 2007]. Selection of the appropriate cell line is based on the
specimen source and the suspected causal viral pathogen. Certain viral pathogens may
require several cell passages before CPEs can be observed. More information on
enumeration assays for viable viruses can be found in standard virology reference texts,
such Principles of Virology [Flint et al. 2009b] and Fields Virology [Fields et al. 2007].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-47 of BA-115
Sampling and Characterization of Bioaerosols
Viral plaque assay
A widely used approach for detecting viral pathogens and quantifying viral titers is the
viral plaque assay (VPA) [Condit 2007]. Under biological safety controls, the
appropriate cell line is propagated and plated, usually in a 6-well format, at a
concentration at which cells form a monolayer. The cell monolayer is next treated for a
defined period (30-60 minutes) with a specific volume (0.1 mL to 1.5 mL) of the
collected bioaerosol and incubated at the specified temperature and CO2 levels for 24
to 72 hours. Throughout the incubation period, cells are routinely inspected and CPEs
are documented. Upon completion of the incubation period, cells are chemically fixed,
stained and plaques (zones of cellular clearing) are enumerated. The final
concentration of the collected viral aerosols is calculated based on the number of
plaques, dilution of the inoculum and volume plated, and is expressed in plaque
forming units per mL (PFU/mL]). While the VPA is a cost-effective method of
assessing sample viral loads, results can take anywhere from 3-5 days. Other
limitations such as the inability to detect low viral titers and inactivated
(noninfectious) virus and the failure of some viruses to form plaques, may
compromise detection and underestimate the viral loads in an aerosol sample.
Likewise, common indoor and outdoor contaminants (such as fungi and bacteria) can
impair the VPA by disrupting the cellular monolayer or outcompeting for nutrients in
the cell culture medium.
Tissue culture infectious dose assay
Another cell culture-based approach to identifying viral aerosols is the Tissue Culture
Infectious Dose assay (TCID50), also known as an endpoint dilution assay [Condit
2007]. As with the VPA, select cells are plated at a desired concentration in a 96-well
format and inoculated with serial dilutions of the collected sample. Following a
specified incubation period, cells are examined for CPEs. The TCID50 is defined as the
dilution of virus required to infect 50% of the cell culture wells [Reed and Muench
1938]. Based on the number of cells that are infected at the designated virus dilution,
viral titers are mathematically calculated. Limitations of the TCID50 are similar to
those observed with the VPA.
Immunofluorescence antibody (IFA) assays
To enhance viral detection and quantify viral loads, immunofluorescence antibody
(IFA) assays (direct or indirect) are frequently used in combination with cell culture-
based methods [Flint et al. 2009a; Tortora et al. 2013]. By combining infected cells
with a fluorescently-labeled, antigen-specific antibody, it is possible to increase
detection levels without the lengthy incubation periods that are typically necessary
with VPAs and TCID50 assays.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-48 of BA-115
Sampling and Characterization of Bioaerosols
c. Interpretation of data
In industrial hygiene surveys that evaluate bioaerosols, indoor bioaerosol levels are usually
compared to outdoor levels or to a control area. In general, indoor bioaerosol levels are
lower than outdoor levels, and the taxa are similar [ACGIH 1989; Burge et al. 1977; Hung
et al. 2005; Macher 1999; Solomon et al. 1980]. However, elevated indoor bioaerosol levels
may be a sign of dampness, water infiltration, or microbial contamination [Hung et al.
2005]. In 2010, The ACGIH published a variety of occupational exposure limits for
aerosols that are derived from biological material in specific industries and include
subtilisins derived from Bacillus subtilis, as well as cotton, grain, flour, wood, and organic
dusts [ACGIH 2015; Eduard et al. 2012]. To date, no occupational exposure limits for
specific fungal bioaerosols exist and these typically fall under particulate matter not
otherwise regulated (10 mg/m3 for inhalable dust; [ACGIH 2015; Eduard et al. 2012].
Proposed limits developed in other regions of the world are provided in Eduard et al.
[2012].
Although the quantification of viable fungal propagules can provide helpful datasets to
evaluate differences between indoor and outdoor fungal diversity, the interpretation of
results should be evaluated closely. Total fungal exposure will be underestimated as non-
viable fungal bioaerosols are not captured in the analysis [Eduard et al. 2012]. Fungal
genera, including Cladosporium, Alternaria, and Epicoccum, and Basidiomycetes are
predominantly localized in outdoor environments and the presence of elevated
concentrations may be an indicator of indoor fungal contamination [Hung et al. 2005].
Similarly, the presence of certain hydrophilic species including Stachybotrys chartarum
and Aspergillus versicolor, and Chaetomium globosum may be signs of indoor fungal
contamination and may require immediate inspection [Flannigan et al. 2011; Hung et al.
2005].
Where local amplification and dissemination of bacteria have not occurred in an occupied,
indoor environment, Gram-positive cocci (e.g., Micrococcus and Staphylococcus) are
normally dominant [Morey et al. 1986]. Airborne human skin scales and respiratory
secretions may contain Gram-positive cocci [ACGIH 1989; Hung et al. 2005]. Detection of
high levels of these microorganisms may be an indication of over-crowding and
inadequate ventilation. Indoor air that tests high for Gram-negative bacteria indicates a
need to identify and eliminate the source of contamination. Concentrations ranging from
4,500-10,000 CFU/m
3
have been suggested as the upper limit for ubiquitous bacterial
aerosols [ACGIH 1989; Nevalainen 1989]. These exposure limits, however, do not apply to
pathogenic microorganisms. Actinomycetes (mesophilic and thermophilic) are commonly
found in agricultural areas. Their presence in indoor environments is an indicator of
contamination [ACGIH 1989; Banaszak et al. 1970; Lacey and Crook 1988]. Thermophilic

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-49 of BA-115
Sampling and Characterization of Bioaerosols
Actinomycetes at concentrations above 70 CFU/m
3
in an affected person's work area have
been regarded as the threshold for triggering remedial action [Otten et al. 1986].
9 Sample analysis methods for non-viable and
non-culturable bioaerosols
The collection and classification of nonviable and non-culturable microorganisms cannot be
performed by using viable culture methods. A large proportion of fungal bioaerosols are non-
viable and would not grow and proliferate on nutrient media [Eduard et al. 2012]. This
fraction of the bioaerosol load is equally important to assess in industrial hygiene surveys that
investigate the role of personal bioaerosol exposure on respiratory health [Brasel et al. 2005;
Green et al. 2011; Mitakakis et al. 2003]. These bioaerosols can also contain antigens,
allergens, microbial volatile organic compounds and even mycotoxins [Brasel et al. 2005;
Eduard et al. 2012; Green et al. 2011; Green et al. 2006b]. Identification of nonviable or non-
culturable microorganisms or components of microorganisms (such as cell wall fragments)
can be performed using a variety of other available assessment strategies such as microscopy,
immunoassays and, more recently, molecular biology techniques [Afanou et al. 2015; Brasel et
al. 2005; Eduard et al. 2012; Flannigan et al. 2011; Green et al. 2011; Hung et al. 2005; Macher
1999; Rittenour et al. 2012].
Microscopy includes a variety of approaches that utilize bright-field, light, phase contrast,
fluorescence or even electron-based approaches [Eduard et al. 2012; Macher 1999]. These
methods enable the enumeration of both viable and nonviable microorganisms [Macher 1999]
as well as other non-culturable bioaerosols including cell wall fragments, plant pollen and
pteridophyte and bryophyte spores [Green et al. 2011; Rittenour et al. 2012]. These
approaches provide a platform to visualize particle morphology and to identify reproductive
fungal structures of individual genera that are based on a combination of propagule
phenotypes [Flannigan et al. 2011; Macher 1999]. However, these approaches can be
confounded by observer bias, especially when it comes to differentiating bioaerosols that
contain similar morphological phenotypes such as amerospores (e.g. Aspergillus conidia)
[Flannigan et al. 2011; Hung et al. 2005]. The ASTM has published a standard method for the
use of optical microscopy to categorize and quantify fungal structures in samples collected by
inertial impaction [ASTM 2009].
To overcome these methodological challenges, alternative methods based on the
quantification of bioaerosol biomarkers (proteins or DNA) have been developed that enable
the quantification of these bioaerosol sources [Eduard et al. 2012]. These include a variety of
assessment methods that can be used to qualitatively and quantitatively assess bioaerosol
exposure by detecting cell wall components, proteins, carbohydrates, or oligonucleotides (e.g.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-50 of BA-115
Sampling and Characterization of Bioaerosols
endotoxin or β-glucan) [Eduard et al. 2012]. Other chemical and proteomic methods
including HPLC, flow cytometry, and mass spectrometry-based approaches can also be used
to detect and quantify cell wall components such as microbial volatile organic compounds and
mycotoxins. These approaches have been reviewed more extensively by Flannigan et al.
[2011].
Several modifications of classical biochemical procedures have been used in recent years to
facilitate inoculation of media, decrease the incubation time, automate the procedure, and
systematize the determination of species based on reaction patterns. Historically, clinical
microbiological techniques have been used for analysis of environmental samples. However,
clinical strains and environmental isolates may differ, requiring modification of clinically-
based techniques.
a. Microscopy
Bright-field or light
In bright-field or light microscopy, an ordinary microscope is used for the
morphological observation and sizing of sampled bioaerosols. Visible light from an
incandescent source is used for illumination and the specimen appears against a bright
backfield. Objects smaller than 0.2 µm cannot be resolved. The image contrast
(visibility) decreases as the refractive index of the substance/microorganism under
observation and the mounting medium become similar. To maximize the contrast, the
mounting medium should have the same refractive index as glass or the immersion oil.
Membrane filters are often "cleared" by using the appropriate immersion oil or acetone
vapor/triacetin combination. This method is commonly used to observe various
stained specimens and to identify and count viable and non-viable bioaerosols. In
addition, pollen grains are often identified and enumerated in this manner [Eduard et
al. 1990].
Collection of fungal bioaerosols onto an adhesive surface followed by microscopic
identification based on the morphological characteristics of the spores (size, shape,
septation etc.) is another common method of assessment. This non-viable method
overcomes limitations introduced in viable analyses and many genera can be
differentiated based on differences in spore morphology [Eduard et al. 2012; Macher
1999]. Microscopic examination of fungi captured on filters or an adhesive tape are
divided into seven spore morphological characteristics and include amero-, didymo-,
helico-, stauro-, dictyo-, phragmo-, and scoleco- spores [Kendrick 2000]. Amerospores
are the most common spore morphology encountered in air samples and are the most
challenging to differentiate taxonomically [Kendrick 2000]. Amerospores are usually
placed in a group represented as Aspergillus/Penicillium group [Hung et al. 2005].
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-51 of BA-115
Sampling and Characterization of Bioaerosols
Many other common environmental fungal bioaerosols share similar morphologies
which can make taxonomic placement challenging for the untrained or inexperienced
observer [Eduard et al. 2012]. Typical magnification used in the assessment of fungal
propagules ranges from 400-1000X. A standard operating procedure for the
assessment of microscopic non-viable samples is presented in Hung et al. [2005] and
by ASTM [2009].
The confounding factors associated with traditional fungal exposure assessment
methods have limited our understanding of the spectrum of fungal bioaerosols in
industrial and occupational environments. Measures using these approaches also
cannot be acquired in real time. Furthermore, identifying and quantifying the
complete diversity of fungal bioaerosols using a standardized methodology is critical
in the determination of fungal bioaerosols within occupational environments [ASTM
2009; Hung et al. 2005].
Phase contrast
Phase-contrast microscopy is used when the microorganism under observation (e.g.,
Escherichia coli) is hyaline and an alternative mounting medium is not possible. As
light passes through the specimen, variations in the index of refraction of the
components cause phase shifts in the light. A phase-contrast microscope uses a special
condenser and diffraction plate that cause these phase shifts to appear as differences in
brightness and contrast. One cannot see an object exactly matching the refractive
index of the mounting liquid; however, very slight differences produce visible images.
This type of microscope is commonly used to provide detailed examination of the
internal structures of living specimens; no staining is required.
Fluorescence
Fluorescence microscopy uses an ultraviolet or near-ultraviolet source of illumination
that causes fluorescent compounds in a specimen to emit light. Fluorescence
microscopy for the direct count of microorganisms has been described in a number of
studies [Eduard et al. 2012]. Direct-count methods to enumerate microorganisms
(especially bacteria) have been developed using fluorescence microscopy and some
stains such as acridine orange, fluorescein isothiocyanate (FITC) and 4’,6-diamidino-
2-phenyl-indole (DAPI) [Macher 1999; Thermo Fisher Scientific 2014]. Utilization of
stains such as calcofluor white can resolve fungal spores and hyphal structures
[Haghani et al. 2013]. This stain binds to chitin; however, other plant-derived and
insect bioaerosol sources (e.g. dust mite, plant pollen) may also be resolved using this
stain. Viability stains also have been developed and are available commercially for the
detection of viable fungi and bacteria bioaerosols in collected air samples [Thermo
Fisher Scientific 2014].
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-52 of BA-115
Sampling and Characterization of Bioaerosols
Electron
Electron microscopy consists of a beam of electrons that enable structures smaller than
0.2 µm, such as viruses, to be resolved. Scanning electron microscopy (SEM) and,
more recently, field emission scanning electron microscopy (FESEM), are approaches
used to study the surface features of prokaryote and eukaryote cells as well as viruses
(usually magnified 1,000-10,000X). These bioaerosols are immobilized onto a semi-
solid filter submicron membrane or in the form of a liquid suspension and a three-
dimensional image of the area is generated. Images from SEM can provide vital
information about the size, morphology and concentration of the collected bioaerosol
[Afanou et al. 2014; Eduard et al. 2012]. However, SEM does not provide information
on viability of the collected bioaerosol. FESEM has been recently used to resolve fungal
fragments that are produced from fungal colonies following abiotic disturbance
[Afanou et al. 2015; Afanou et al. 2014]. Transmission electron microscopy can also be
used to examine viruses or the internal ultrastructure in thin sections of cells (usually
magnified 10,000-100,000X), although the image produced is not three- dimensional.
Compared to other methods of assessment described in this chapter, SEM and
FESEM-based approaches require a highly trained technician to obtain images from
bioaerosols captured on filter membranes.
b. Endotoxin assays
The lipopolysaccharide endotoxin is a virulence factor possessed by all Enterobacteriaceae
(as well as other Gram-negative bacteria) that is found in the outer membrane of the cell
wall. Airborne endotoxin has been found in high concentrations in agricultural, industrial,
and office environments [Eduard et al. 2012; Milton et al. 1990; Rylander and Vesterlund
1982; Singh et al. 2011b]. Individuals may experience disseminated intravascular
coagulopathy, respiratory tract problems, cellular and tissue injury, fever, and other
debilitating problems. Endotoxin can be detected in air samples collected on glass filters
using the Limulus amebocyte lysate (LAL) assay [Eduard et al. 2012]. This assay uses
amebocytes from the blood-like circulating fluid of the Limulus polyphemus (horseshoe
crab). After exposure to the lysed amebocyte cells, the chromogenic version of the LAL
enables endotoxins to be quantified [Eduard et al. 2012]. Laboratories use this assay to test
for contamination by Gram-negative bacteria [Baron and Finegold 1990].
Although widely used, endotoxin aerosol measurement techniques lack comparability
between results obtained in different laboratories because of differing sampling,
extraction, and analytical methods [Jacobs 1989; Milton et al. 1990; Olenchock et al. 1983;
Rylander and Vesterlund 1982] and water-insoluble endotoxins are not detected [Eduard
et al. 2012]. A monoclonal antibody assay has also been developed but it is less sensitive

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-53 of BA-115
Sampling and Characterization of Bioaerosols
than the LAL method. Similarly, chemical-based approaches are available to detect
endotoxins including gas chromatography-mass spectrometry [Eduard et al. 2012].
c. Biochemical analysis methods
Because of the high frequency of isolation of Gram-negative rods in clinical settings,
several commercial multi-test systems have been developed for identification of members
of the family Enterobacteriaceae and other pathogenic microorganisms. These
microorganisms are indistinguishable except for characteristics determined by detailed
biochemical testing. These systems require that a pure culture be examined and
characterized. A list of some commercially available identification kits is provided in Table
III. All of these multitest systems have documented accuracies greater than 90% in clinical
settings [Baron and Finegold 1990; Koneman 1988]. For fungi, API (Analytab Products,
Plainview, NY) and BIOLOG can also be used to differentiate yeasts based on the
respective biochemical and physiological profiles [Flannigan et al. 2011].
d. Chemotaxonomic approaches
Cellular fatty acids (CFA) of bacteria are structural in nature, occurring in the cell
membrane or cell wall of all bacteria. When the bacteria are grown under standardized
growth conditions, the CFA profiles are reproducible within a genus, down to the
subspecies or strain level in some microorganisms. The Sherlock Microbial Identification
System (MIS), developed by MIDI (Newark, DE), provides a chromatographic technique
and software libraries capable of identifying various microorganisms based on their CFA
composition [Sasser 1990a; Sasser 1990b]. The chromatographic technique is also known
as gas chromatography fatty acid methyl ester analysis (GC-FAME). MIS has a database
containing the analysis libraries for culturable Gram-negative and Gram-positive bacteria,
and yeasts. In a comparison study [Amy et al. 1992], only 8 of 18 isolates, identified by
either API multitest or MIDI MIS, were identified accurately using BIOLOG multitest. A
prototype method for extracting and analyzing fungi is currently being distributed by
MIDI.
e. Chemical-based approaches
A variety of chemical-based approaches are available for the detection and quantification
of bacteria and fungi in environmental samples [Flannigan et al. 2011; Hung et al. 2005].
Common approaches include thermal desorption - gas chromatography - mass
spectrometry (TD-GC-MS), high performance liquid chromatography, gas
chromatography-tandem mass spectrometry, and matrix-assisted laser
desorption/ionization time of flight (MALDI-TOF) mass spectrometry [Flannigan et al.
2011]. These methods require the formation of a library of markers or spectral signatures
that are used to discriminate between various prokaryote and eukaryote species. Examples
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-54 of BA-115
Sampling and Characterization of Bioaerosols
of spectral signatures that have been used for the detection of fungi include microbial
volatile organic compounds (mVOCs), mycotoxins, ergosterol, 3-hydroxy fatty acids,
muramic acid as well as intracellular and extracellular proteins. NIOSH Method 2549 is a
TD-GC-MS based approach that allows for the detection of mVOCs in environmental
samples [NIOSH 2003d].
f. High performance liquid chromatography
High performance liquid chromatography (HPLC) is commonly used for bioaerosol
“fingerprinting” and biomass determination. Techniques such as proteomics and
identifying variations in chromatographic patterns can be used to determine the source of
airborne material. For example, ergosterol has been used for decades to detect fungal
contamination [Seitz et al. 1979] and even determine taxonomy [Axelsson et al. 1995;
Pasanen et al. 1999; Schnurer 1993]. Keratin analysis can be used to identify bioaerosols
derived from vertebrates and possibly the habitat within an environment [Staton et al.
2013]. Detection can be as straightforward as using UV absorbance or as complex and
specific as employing an ion trap mass spectrometer.
HPLC can be adventitious compared to other methods of analysis; it is an established
technology, fairly inexpensive after initial equipment costs, fast and accurate. Its
disadvantages include a lack of specificity inherent with detectors using UV absorbance
(especially at lower wavelengths), and that complex matrices associated with bioaerosols
can prove to be troublesome and may require multi-step enhancement procedures such as
solid-phase extraction. Buffered solvent systems are sometimes required, which can be
technically difficult to use.
Mycotoxins
Fungal contamination is a concern in food production because it can modify the
nutritional content of feed and cereal grains and introduce potentially adverse
mycotoxins. Before the use of HPLC, methods of identifying fungal contamination
were time consuming or missed non-viable organisms which still contributed to the
biomass [Seitz et al. 1979]. HPLC can be used to detect ergosterol, which is a structural
sterol nearly universally present in fungi but not naturally present in grains [Pasanen
et al. 1999]. HPLC has also been used to detect ergosterol, mannitol and arabitol in
bioaerosols [Buiarelli et al. 2013].
Ergosterol is extracted using a liquid-liquid extraction and concentrated. The clean
samples are then analyzed by HPLC with UV detection. The ergosterol UV spectrum
varies significantly from the UV spectrum of higher plant sterols, making it specific to
fungal contaminants. Though the method is still fairly time consuming, it eliminates
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-55 of BA-115
Sampling and Characterization of Bioaerosols
the need for fungal culturing and can detect the presence of non-viable fungi. When
combined with other sterols this information can be used to help determine fungal
species [Schnurer 1993].
In addition to using sterols to identify fungal contamination, mycotoxins can also be
analyzed by liquid chromatography with mass spectrometry (LC-MS). Identification of
specific mycotoxins can help identify the species of fungi present [Bennett and Klich
2003; Castillo et al. 2016].
Mycotoxins can play a role in indoor air quality (IAQ), food safety and possibly
bioterrorism. The use of LC-MS as a screening tool to identify mycotoxins can reduce
the use of more intensive molecular techniques for identification and quantitation.
When using mass spectral analysis it is important to have a reliable and accurate
database for identification and a qualified analyst to correctly interpret data.
Other biomolecules
More recently, HPLC has been proposed as a forensic tool to identify and track
vertebrate species using keratin profiles. Like ergosterol in fungi, keratin is found
mainly in dander left by vertebrates [Plowman 2007]. If patterns can be established, it
may be possible to identify what species had been present in a specific dwelling and
possibly even track movements [Staton et al. 2013]. Bacterial contamination can also
be tracked using endotoxin analysis and bacterial peptidoglycan fingerprinting [Staton
et al. 2013].
Sample preparation and enhancement
Bioaerosols can have complex matrices with many interfering constituents. Ultraviolet
absorption detectors are fairly inexpensive and straightforward to use, but they can
suffer from a lack of specificity and sensitivity. Although mass spectrometry detectors
do not suffer from a lack of specificity or sensitivity, a dirty sample can still present
challenges.
Another potential pitfall is the presence of large particles in bioaerosol samples. In
general, analytical HPLC systems and detectors use small diameter tubing and small
orifice injectors that are easily clogged or contaminated by particles. Most analytical
systems have some sort of less-expensive trapping or pre-column that can be sacrificed
in order to spare the more expensive analytical columns. However, trap columns are
still very much an expense and should only be considered if other options are not
available. A multitude of cleanup procedures are available to lessen or eliminate
problems due to particles, the most common of which are simple centrifugation and
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-56 of BA-115
Sampling and Characterization of Bioaerosols
filtration. Both methods will lessen the likelihood of clogging or damaging the system,
but neither offers target analyte enrichment.
Liquid-liquid extraction
In order to enrich analytes, samples can be concentrated, chemical interferences can be
removed, or a combination of both methods can be used. One of the oldest methods of
enrichment is a liquid-liquid extraction, which separates analytes based on relative
solubility in a given solvent [Koncsag and Barbulescu 2011]. In general, two
immiscible solvents are mixed, one solvent containing the whole extract (called the
“feed”) and one that ideally solubilizes the analyte of interest preferentially. Based on
solubility, the chemical constituents will either stay in the feed solvent or partition into
the other solvent. Once the solvents are allowed to phase-separate, the solvent
containing the enriched analyte (called the raffinate) is removed.
Liquid-liquid extraction is usually done using an aqueous solvent and an organic
solvent. Solvent selection is critical and can be difficult. The goal of the extraction is to
choose a solvent that leaves behind as much of the interfering matrix as possible in the
feed solvent while also being able to preferentially solvate the analyte of interest. The
solvent also must be compatible with the analytical instrumentation. Liquid-liquid
extractions tend to use large amounts of solvent, which can result in the analyte of
interest being below analytical levels of detection and/or levels of quantitation. Many
organic solvents can be easily concentrated by evaporation, but this can pose problems
with labile or volatile analytes.
Solid-phase extraction
Another option that can help avoid these pitfalls is the use of solid-phase extraction
(SPE) [Sigma-Aldrich 1998]. SPE exploits the analyte affinity (or lack thereof) for a
solid material packed inside a column. Sample enrichment can occur by the column
retaining interfering compounds and the analyte of interest passing through, or by the
column retaining the analyte and the interfering chemicals passing through.
Concentration of the analyte of interest can be achieved by eluting the analyte in a
smaller volume of solvent, and filtration can be achieved simultaneously. SPE may
make it possible to achieve analyte concentration and avoid potential losses that could
arise from concentrating a large volume of solvent. Appropriate solvent selection is
also critical to successful SPE.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-57 of BA-115
Sampling and Characterization of Bioaerosols
g. Immunoassays
The immunoassay is an analytical technique for measuring a targeted antigen, which is
also referred to as an analyte. A critical component of the immunoassay is the antibody or
ligand, which binds a specific antigen or binding site. The binding of the antibody or
ligand forms the basis for the immunoassay, and numerous formats have been devised
which permit visual or instrumental measurements of this reaction. Antibodies include
either monoclonal or polyclonal antibodies and these are commonly employed to detect
organisms by binding to antigens, usually proteins, polysaccharides or other cell wall
components [Hung et al. 2005; Macher 1999]. The analysis is usually performed following
extraction of the analytes to form a heterogeneous matrix. In most immunoassays, there is
little need for extensive sample cleanup. Following the development of
radioimmunoassays, many immunoassays that use monoclonal antibodies are now readily
available from commercial sources, permitting laboratories to use standardized
immunoassays or rapidly develop in-house immunochemical assays. In addition,
commercially available immunoassays or multiplex platforms are available to quantify a
variety of indoor or occupational bioaerosol sources [King et al. 2013]. Some of the more
widely used immunoassay formats are as follows:
Enzyme immunoassays (EIA)
Enzyme Immunoassays (EIA) are composed of a variety of assay formats that can be
used to quantify bioaerosols in an air or dust sample. The binding of an antibody or
antigen to an enzyme, such as horseradish peroxidase (HRP) or alkaline phosphatase
(AP), is the basis of EIA techniques. Enzymatic activity, in the presence of a
chromogen, results in a colored end-product that is quantified using a
spectrophotometer. Many, if not most, commercially available EIAs are enzyme-linked
immunosorbent assays (ELISAs). There are four types of ELISAs which include direct
ELISA, indirect ELISA, sandwich ELISA and competitive ELISA.
The sandwich ELISA method is typically used for the detection for airborne viruses
and aeroallergens [Hung et al. 2005]. In this assay, a capture antibody is bound to a
solid surface, usually a 96-well plate. The bioaerosol extract is added to the plate
containing the capture antibody and incubated for a specified length of time, washed
with a phosphate buffer solution and probed with an enzyme-labeled antibody that
enables detection and quantification, either through colorimetric changes or
fluorescence emissions. The advantages to using a sandwich-based ELISA method are
that it is highly specific and can be used on complex samples such as aerosols. Some
disadvantages to using a sandwich-based ELISA are poor antibody recognition and/or
minimal detection due to low sample concentration. Lastly, it should be noted that
ELISA, like any protein-based assay, does not distinguish between viable and non-
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-58 of BA-115
Sampling and Characterization of Bioaerosols
viable viral aerosols. Multiplexed technologies that enable the detection and
quantification of multiple allergen sources in one sample are also available from a
variety of commercial sources [King et al. 2013].
EIA methods to assess fungal bioaerosols based on the detection of fungal cell wall
components, enzymes, antigens and allergens have become commercially available. β-
1,3-D glucan, extracellular polysaccharides, mycotoxins and a variety of fungal
antigens can now be quantified in air and dust samples using immunochemical,
enzymatic and chemical detection platforms [Chew et al. 2001; Douwes et al. 1997;
Eduard et al. 2012]. Although many of these biomarkers and methods serve as a proxy
measure for total fungal biomass, these approaches can be confounded by limitations
associated with component extraction biases. In addition, complex extraction,
washing, amplification or immunochemistry steps are required that may add hours or
even days before a dataset is finalized for analysis and interpretation.
To date, there are a number of commercial companies that have developed ready to
use EIA kits for the detection and quantification of a variety of indoor and
occupational biomarkers including dog, cat, dust mite, fungal and rodent allergens
[Filep et al. 2012]. These EIA approaches enable the collection and quantification of
these biomarkers in the work environment and provide a ready to use platform for the
industrial hygienist.
Fluorescent immunoassays (FIA)
Utilization of fluorescent-labeled antibodies to detect bacterial antigens was
introduced by Coons et al. [1941; 1942]. Various FIA techniques have now evolved
and are commonly utilized in laboratories. These include: (1) direct FIA, to detect cell-
bound antigens using a fluorescent antibody; (2) indirect FIA to detect cell-bound
antigens using a primary antibody and a fluorescent secondary antibody; and (3)
indirect FIA to detect serum antibodies using an antigen, serum, and a fluorescent
antibody. Various fluorescent dyes, such as fluorescein, fluorescein isothiocyanate, and
rhodamine isothiocyanate, may be employed [Thermo Fisher Scientific 2014]. A
fluorescent or confocal microscope is used to evaluate the samples and to count the
number of fluorescently stained organisms [Garvey et al. 1977; Popp et al. 1988].
Similarly, flow cytometry-based approaches using fluorescent-labelled antibodies have
also been employed to evaluate a variety of bioaerosol sources including bacteria,
pollen and fungi [Rittenour et al. 2012; Rule et al. 2007; Rydjord et al. 2007].
Multiplexed approaches have been developed for the detection of multiple allergens in
the same extracted sample [King et al. 2013].
FIA can be used to detect viruses. An example of a direct FIA assay for the detection of
virus-laden aerosols is the Focus Forming Assay (FFA) [Flint et al. 2009a]. With the

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-59 of BA-115
Sampling and Characterization of Bioaerosols
FFA, fluorescent microscopy is used to visualize immunostained cells and viral titers
are quantified as focus forming units per milliliter, or FFU/mL. While the FFA is more
sensitive than culture-based methods alone, samples with low viral titers may weakly
fluoresce and possibly be considered undetectable. To overcome weak detection levels,
an indirect IFA assay may be more appropriate [Leland and Emanuel 1995; Madeley
and Peiris 2002]. With indirect IFA assays, a primary, unconjugated antibody is used
in combination with a fluorophore-conjugated, secondary antibody directed against
the primary antibody. Because the secondary antibody is able to bind to multiple
epitopes on the primary antibody, it increases fluorescence and enhances overall
detection. While IFA is a trusted method of viral detection and quantification, it is
fraught with limitations including excessive cost and the necessity of a skilled
technician experienced in the reading of immunofluorescence. Likewise, because
viruses are constantly undergoing antigenic drift and occasionally antigenic shift,
changes in viral antigens can affect the binding affinity of the primary antibody and
may result in false negatives.
Ligand-based assays
As an alternative to cell culture and immunofluorescent-based assays, there are several
protein-based methods of detection that can be used to quantify viral loads in an
aerosol sample. The hemagglutination (HA) assay is a non-fluorescence quantitative
assay that is based upon the ability of certain viral pathogens to agglutinate species-
specific erythrocytes [Condit 2007]. In a serial twofold dilution, viral samples are
mixed with a 1% solution of erythrocytes and incubated at room temperature for 30-
60 minutes. Viral samples which form an agglutinated lattice are able to prevent red
blood cells from precipitating out of solution by the binding of the hemagglutinin
protein (present on the surface of the viral pathogen) to the sialic acid receptors
(present on the surface of red blood cells). The titer of the sample is based on the well
with the last agglutinated appearance, immediately before the well in which the red
blood cells have settled out of solution. Hemagglutination units (HAUs) are typically
used to quantify the viral concentration.
One of the major limitations of the HA assay is that it does not distinguish between
infectious and non-infectious viral particles. Likewise, certain bacteria and fungi
possess hemolytic activity and when present in a collected bioaerosol, can alter the HA
assay and result in false positive readings. To circumvent this issue, a variation of the
HA assay, known as the Hemagglutination-Inhibition test, can be performed [Stewart
et al. 1967]. The HA inhibition test measures serum antibodies that are directed
against the viral pathogen. When present in sufficient concentration, the serum
antibodies are able to prevent agglutination of red blood cells thereby providing an
alternative means of quantifying viral loads.
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-60 of BA-115
Sampling and Characterization of Bioaerosols
Direct and indirect immunostaining
Direct and indirect immunostaining methods have been previously described for the
detection of bioaerosol sources. Popp et al. [1988] developed a staining technique to
enumerate bioaerosol samples directly captured on microscope slides. These
approaches have enabled the identification of specific bioaerosol sources, especially
those that do not contain morphological phenotypes that would be used by a trained
microbiologist to resolve and identify a specific microorganism [Popp et al. 1988].
Alternatives to this approach have been developed and utilized in a variety of indoor
and occupational environments. A press blotting approach that included immobilizing
proteins from collected bioaerosols captured on an adhesive tape provided insight into
the bioaerosols that contain allergen in the outdoor environment [Takahashi et al.
1993; Takahashi and Nilsson 1995]. An alternative method, called the Halogen
Immunoassay, enables the immunostaining of allergen and antigen around bioaerosols
captured on a protein binding membrane such as PVDF or mixed cellulose ester
[Green et al. 2006c; Tovey et al. 2000]. This immunoassay approach has been used in a
variety of indoor and occupational settings to evaluate allergen sources including, cat,
dog, latex, rodent, plant, and fungi [Green et al. 2006a; Green et al. 2003; Green et al.
2005a; Green et al. 2011; Green et al. 2005b; Green et al. 2005c; Green et al. 2006b;
Green et al. 2006c; Mitakakis et al. 2001; Poulos et al. 2002; Poulos et al. 1999;
Razmovski et al. 2000; Renstrom 2002; Tovey and Green 2004]. Recently these
approaches have been adapted to FESEM applications and have been used to detect
morphologically indiscernible fungal fragments [Afanou et al. 2015].
Biosensors
To overcome some of the technical challenges associated with traditional methods to
assess bioaerosol exposure, real-time sensor technologies are being developed for the
detection of bioaerosols [Fronczek and Yoon 2015; Hook-Barnard et al. 2014]. The
sensor technologies are based on a variety of signal detection strategies that include
optical, mechanical, electrical, or magnetic sensing approaches. These methods have
resulted in platforms that have enabled the rapid detection of microbial pathogens and
in an automated format. Using this technology requires little technological skill and
can be developed into an automated handheld device. These developments have
resulted in the fabrication of remote monitoring units in the agricultural sector that
can process data remotely and apply it to geographical information systems or
forecasting models.
Biosensors have been developed for a variety of applications including the detection of
microbial pathogens [Fronczek and Yoon 2015; Hook-Barnard et al. 2014]. Typically,
a biologically derived analyte (such as fungal spores, hyphae, antigens, peptides or
nucleotides) is collected and interacts with a selected bioreceptor immobilized on a

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-61 of BA-115
Sampling and Characterization of Bioaerosols
sensor surface. Examples of bioreceptors include oligonucleotides,
monoclonal/polyclonal antibodies, enzymes, cells, and even phages. The bioreceptor
system consists of a physiochemical transducer that produces a measurable signal.
Transducers can be optical and include surface plasmon resonance (SPR), which is a
method that measures changes in the refractive index during molecular binding events
[Unser et al. 2015; Usachev et al. 2014]. In contrast, electrochemical transducers
measure changes in current, potential, impedance, and conductance across an
electrode surface for detection events [Patolsky et al. 2006]. Examples of
electrochemical detection include the measurement of electrical conductance
produced by antibody-antigen binding events [Patolsky et al. 2004]. Both optical and
electrochemical transducers have been developed for the detection of a variety of
pathogens in the biosecurity, medical and agricultural sectors.
h. Gene-based assays
Cell culture, protein-based, and immunological-based assays are invaluable diagnostic
tools. However, the evolution of nucleic acid-based molecular diagnostics are rapidly
becoming the preferred method for detecting and quantifying bioaerosols [West et al.
2008]. Nucleic acid-based molecular diagnostics can be divided into two categories, (1)
Direct sample analysis and (2) Indirect sample analysis, such as the Viral Replication
Assay (VRA), which requires cultivation of the target microorganism prior to molecular
analysis [Blachere et al. 2011]. Regardless of which approach is taken, there are three steps
involved: extraction and purification of nucleic acids; amplification of the gene target; and
detection of the amplicon. For viral, bacterial, plant and fungal nucleic acid extraction and
purification, a number of kits are commercially available. Such kits generally rely upon
either silica adsorption (spin-column) or affinity purification (magnetic separation)
methodologies. Because bioaerosols are typically dilute in nature, investigators should
determine which method yields the greatest amount of nucleic acids while minimizing
sample handling, contamination and degradation. These are important variables to
consider and can vary depending on the selected approach.
With the advent of molecular assays such as the Polymerase Chain Reaction (PCR) and
Real-Time Quantitative PCR (RT-qPCR), which detect specific genetic sequences in the
sample DNA or RNA, it has been possible to provide standardized assays, reduce
turnaround time, and enhance assay sensitivity and detection specificity [Cella et al. 2013;
Life Technologies 2014; Mahony 2008]. Using gene-specific oligonucleotides coupled with
either an intercalating fluorescent dye (e.g. SYBR green) or a fluorogenically labeled gene
probe (e.g. VIC, 6FAM), industrial hygienists are able to monitor indoor and outdoor
bioaerosols for the presence of microorganisms. Likewise, multiplexing PCR and bead-
based multiplexing PCR, which couples PCR and flow-cytometry, can be used for high-

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-62 of BA-115
Sampling and Characterization of Bioaerosols
throughput screening of multiple respiratory viruses in a single reaction mixture. It should
be noted that poor assay design, primer and probe base-pair mismatches and degraded
template nucleic acids can lead to false negatives or reduced detection sensitivity.
Investigators should ensure optimal assay design, validate limits of detection, and run
valid PCR controls in parallel.
Within the last two decades, a variety of molecular technologies have been used to
quantify eukaryotic biomass including fungi and plant pollen in occupational, health,
residential, and industrial samples [Rittenour et al. 2012; Scott et al. 2011; Summerbell et
al. 2011]. Examples of these technologies include molecular based methods to evaluate
specific or conserved gene loci (internal transcribed spacer region of ribosomal RNA) such
as Sanger [Rittenour et al. 2014] or next generation sequencing [Kettleson et al. 2015],
denaturing gradient gel electrophoresis [Johansson et al. 2014] and quantitative PCR
[Eduard et al. 2012; Vesper et al. 2007]. The latter approach includes examples such as a
DNA-based mold specific quantitative PCR (msQPCR) method that enables the detection
and quantification of 36 indicator fungal species [Vesper et al. 2007]. This msQPCR
method has been used to develop an Environmental Relative Moldiness Index (ERMI) to
quantify the mold burden in homes. Originally developed by the U.S. Environmental
Protection Agency (EPA) [Vesper et al. 2007], this approach has been licensed to a variety
of companies in the commercial sector and is widely used to evaluate indoor fungal
bioaerosol particles in settled dust during investigations of indoor air quality [Bolaños-
Rosero et al. 2013; Kettleson et al. 2015; Reponen et al. 2012; Reponen et al. 2011a; Taubel
et al. 2016; Vesper et al. 2013; Vesper et al. 2007]. The development of this methodology
has provided the first step towards a standardized approach to quantify fungal bioaerosol
sources within the indoor environment. Other metagenomic molecular methods including
Sanger, 454, and Illumina miSeq sequencing platforms have also provided new insights
into the complete diversity of bacterial and fungal bioaerosols in indoor, outdoor and
occupational environments.
10 Limitations of bioaerosol sampling and
characterization
Bioaerosol sampling can be a useful tool to study occupational exposures, potential health
hazards, and the transmission of infectious diseases. However, bioaerosol sampling has
significant limitations, and these need to be kept in mind when deciding whether or not to
collect bioaerosol samples, preparing a sampling plan, and interpreting the results.
The first and most important limitation is the lack of standards and guidelines for acceptable
bioaerosol exposure limits. NIOSH and other organizations have set recommended exposure

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-63 of BA-115
Sampling and Characterization of Bioaerosols
limits for several organic materials which may contain microorganisms and their fragments,
such as cotton dust, grain dust, starch and wood dust [NIOSH 2010]. Although numerous
studies have suggested a connection between exposure to various bioaerosols and respiratory
illnesses, exposure limits do not currently exist in the US for airborne pollen, fungi, protozoa,
bacteria, viruses, or their fragments. These limits have not been established largely because it
is not possible to definitively state that a particular bioaerosol concentration will or will not
lead to adverse health outcomes [Eduard et al. 2012; Heederik 2013; Morey 2007; Nevalainen
et al. 2015; NIOSH 2012a]. This is true for several reasons: bioaerosols are often a complex
mixture of microorganisms and organic materials; thousands of species of microorganisms
exist, and most have not been studied; microorganisms and their fragments can cause illnesses
in a variety of ways, including allergic reactions, infections and toxicity; the health effects of
biological materials can vary substantially from person to person; and sampling and analytical
procedures are not standardized, which makes it difficult to compare results [Eduard et al.
2012; Heederik 2013; Morey 2007; Nevalainen et al. 2015; Taubel et al. 2016]. Although
research is ongoing, no standards for acceptable levels of bioaerosols in the environment have
been established by the US government or organizations such as the ACGIH or the AIHA.
In addition to the lack of occupational exposure limits for bioaerosols, measuring and
interpreting bioaerosol concentrations are more complex than is often appreciated. Bioaerosol
concentrations can vary significantly from location to location within a building, especially if
the bioaerosol has one or a few localized sources. A study of bioaerosol exposure in a large
engine plant found that levels of airborne fungi, bacteria and endotoxin varied from location
to location within the plant [Thorne et al. 1996]. A study of airborne fungi in two residences
found significant differences between two rooms sampled at the same time [Hyvarinen et al.
2001]. In healthcare settings, patients with certain respiratory infections expel bioaerosol
particles containing infectious pathogens. Because of the dispersion of the aerosol and the
settling of larger droplets, the bioaerosol concentration decreases rapidly as the distance from
the patient increases [Jones and Brosseau 2015]. A study of airborne influenza in a healthcare
clinic found that the concentrations were much higher in examination rooms containing
patients with influenza than other locations, and that the airborne influenza concentration
also varied from location to location within the waiting room [Lindsley et al. 2010a].
Most bioaerosol collection methods provide a snapshot of the environmental bioaerosols at a
specific time. Temporal variations in bioaerosol concentrations are commonly observed,
especially if the bioaerosol generation occurs during episodic events rather than continuously.
One study of indoor airborne mold in a residence found that day-to-day concentrations of
airborne fungi varied considerably, and that levels were 26 times higher in the summer than in
the winter [LeBouf et al. 2008]. Another residential study found that more airborne fungi were
present in the morning than the afternoon and earlier in the winter compared to later
[Hyvarinen et al. 2001]. In the influenza study mentioned above, day-to-day levels of airborne

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-64 of BA-115
Sampling and Characterization of Bioaerosols
influenza virus varied considerably depending upon the number of influenza patients present
[Lindsley et al. 2010a].
Other factors also influence bioaerosol concentrations. Building airflow patterns and the
operation of the HVAC system can affect bioaerosol levels, particularly if the HVAC system is
a source of bioaerosol particles [Macher 1999]. Areas occupied by people show increased
levels of bioaerosols compared to empty spaces, both because people themselves shed
bioaerosol particles and because human activities such as walking and sitting can re-suspend
dust from floors and furniture [Buttner and Stetzenbach 1993; Ferro et al. 2004; Hung et al.
2005; Qian et al. 2012]. Outdoor air is an important source of airborne fungi in many indoor
environments due to fresh air being drawn in by HVAC systems and infiltration through
cracks and openings [Eduard 2009].
Because of these issues, if bioaerosol sampling is to be conducted, it needs to be a part of a
well-planned and comprehensive sampling strategy. The development of a sampling plan
should begin with a thorough inspection and understanding of the workplace, including the
building, HVAC system, and possible sources of bioaerosols. The sampling plan should
integrate other types of data collection with the bioaerosol sampling, such as bulk sampling of
possible source materials, surface sampling of settled dust, and health surveys of workers.
Collections will need to be carried out at multiple locations and multiple time points, and
even then it must be kept in mind that such samples may not fully characterize the exposure
and that false negative results are quite possible [ASTM 2014a; Hung et al. 2005; Macher 1999;
Morey 2007].
Bioaerosol sampling can be beneficial when done in the appropriate context [Hung et al. 2005;
Macher 1999; Morey 2007]. It can be helpful to compare indoor and outdoor levels of
bioaerosols to identify possible indoor problem microorganisms. Sampling for specific
microorganisms of concern can be useful, especially if there is a known source, such as a
composting operation or an aeration tank in a sewage treatment facility [Environment Agency
2009; Masclaux et al. 2014]. Bioaerosol sampling is also a valuable research tool for better
understanding sources and exposures. On the other hand, sampling for bioaerosols will not be
helpful if there is not some basis for interpreting the resulting data. For example, because of
the lack of dose-response information and the variability associated with bioaerosol sampling,
NIOSH does not recommend routine air sampling when investigating possible respiratory
illness due to exposures in damp buildings. Instead, NIOSH recommends inspections of the
building and its HVAC systems to locate moisture and microbial growth problems, followed
by remediation [NIOSH 2012a].

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-65 of BA-115
Sampling and Characterization of Bioaerosols
11 Safety considerations
Investigators should use appropriate personal protective equipment (PPE) and practice good
personal hygiene when conducting indoor environmental quality, disease outbreaks, and
agricultural health investigations that have resulted in medically diagnosed symptoms. PPE
may include respiratory protection to prevent inhalation of microbes and microorganism-
resistant clothing to prevent transmission to investigators by bodily contact with
microorganisms. Good personal hygiene practices include washing exposed skin and clothing
thoroughly and refraining from eating, drinking, or smoking in a contaminated area. These
simple steps will help minimize the ingestion, inhalation, or uptake of microorganisms.
All samplers, culture plates, and other equipment should be handled aseptically to prevent
contamination of the samples and, more importantly, to prevent the spread of potential
human pathogens to the worker or the work environment. All surfaces, including washed
hands, may harbor microorganisms or spores unless they are specifically sterilized. Practically
speaking, however, not all objects can be sterilized. While disinfection with an oxidizing
chemical or alcohol destroys most vegetative cells, these agents do not destroy all spores.
Samplers should be disinfected or, if possible, sterilized after each sample collection. Special
care should be given to samplers with convoluted inlets or air pathways where
microorganisms may accumulate.
Information on the safe handling of biological specimens can be found in in the free online
manual “Biosafety in Microbiological and Biomedical Laboratories” from the Centers for
Disease Control and Prevention at
(http://www.cdc.gov/biosafety/publications/bmbl5/index.htm) [CDC 2009]. Information on
the handling of some specific pathogens can also be found at the CDC website (www.cdc.gov).
12 Resources
The NIOSH Manual of Analytical Methods has several chapters discussing other aspects of
aerosol sampling, including general considerations and factors affecting aerosol sampling, an
explanation of filter pore size, sampling airborne fibers, sampler wall losses, and avoiding
bypass leakage in filter cassettes [NIOSH 2003e]. NIOSH also maintains a web page on indoor
environmental quality with more information and links to additional resources at
http://www.cdc.gov/niosh/topics/indoorenv/. The American Industrial Hygiene Association
(AIHA; https://www.aiha.org) has reference materials and resources on indoor air quality and
bioaerosols, as does the American Conference of Governmental Industrial Hygienists
(ACGIH; http://www.acgih.org). ASTM International (http://www.astm.org) publishes
numerous standards and guides on the evaluation of indoor air quality, including developing

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-66 of BA-115
Sampling and Characterization of Bioaerosols
an air sampling strategy and the collection and evaluation of bioaerosols [ASTM 2009; ASTM
2014a; ASTM 2014b; ASTM 2014d; ASTM 2014e]. The Centers for Disease Control and
Prevention has guidelines on environmental infection control in healthcare facilities that
include recommendations on environmental sampling [CDC 2003].
Disclaimer
Mention of any company or product does not constitute endorsement by NIOSH. In addition,
citations to websites external to NIOSH do not constitute NIOSH endorsement of the
sponsoring organizations or their programs or products. Furthermore, NIOSH is not
responsible for the content of these websites. All web addresses referenced in this document
were accessible as of the publication date.
13 References
Abed Y, Boivin G [2006]. Treatment of respiratory virus infections. Antiviral Res 70(2):1-16.
ACGIH [1989]. Guidelines for the assessment of bioaerosols in the indoor environment.
Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
ACGIH [2001]. Documentation of the Threshold Limit Values and Biological Exposure
Indices. Appendix C: Particle size-selective sampling criteria for airborne particulate matter.
Cincinnati, Ohio: American Conference of Governmental Industrial Hygienists.
ACGIH [2015]. TLVs and BEIs based on the documentation of the Threshold Limit Values
for chemical substances and physical agents and Biological Exposure Indices. Cincinnati, OH:
American Conference of Governmental Industrial Hygienists.
Adams RI, Tian Y, Taylor JW, Bruns TD, Hyvarinen A, Taubel M [2015]. Passive dust
collectors for assessing airborne microbial material. Microbiome 3:46.
Adhikari A, Reponen T, Rylander R [2013]. Airborne fungal cell fragments in homes in
relation to total fungal biomass. Indoor Air 23(2):142-147.
Afanou KA, Straumfors A, Skogstad A, Nayak AP, Skaar I, Hjeljord L, Tronsmo A, Eduard W,
Green BJ [2015]. Indirect immunodetection of fungal fragments by field emission scanning
electron microscopy. Appl Environ Microbiol 81(17):5794-5803.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-67 of BA-115
Sampling and Characterization of Bioaerosols
Afanou KA, Straumfors A, Skogstad A, Nilsen T, Synnes O, Skaar I, Hjeljord L, Tronsmo A,
Green BJ, Eduard W [2014]. Submicronic fungal bioaerosols: high-resolution microscopic
characterization and quantification. Appl Environ Microbiol 80(22):7122-7130.
Amy PS, Haldeman DL, Ringelberg D, Hall DH, Russell C [1992]. Comparison of
identification systems for classification of bacteria isolated from water and endolithic habitats
within the deep subsurface. Appl Environ Microbiol 58(10):3367-3373.
Andersen AA [1958]. New sampler for the collection, sizing, and enumeration of viable
airborne particles. J Bacteriol 76(5):471-484.
Appert J, Raynor PC, Abin M, Chander Y, Guarino H, Goyal SM, Zuo ZL, Ge S, Kuehn TH
[2012]. Influence of suspending liquid, impactor type, and substrate on size-selective sampling
of MS2 and adenovirus aerosols. Aerosol Sci Technol 46(3):249-257.
Arantes TD, Theodoro RC, Da Graca Macoris SA, Bagagli E [2013]. Detection of
Paracoccidioides spp. in environmental aerosol samples. Med Mycol 51(1):83-92.
Artenstein MS, Miller WS, Lamson TH, Brandt BL [1968]. Large-volume air sampling for
meningococci and adenoviruses. Am J Epidemiol 87(3):567-577.
Artenstein MS, Miller WS, Rust JH Jr., Lamson TH [1967]. Large-volume air sampling of
human respiratory disease pathogens. Am J Epidemiol 85(3):479-485.
Ashley K, Harper M [2013]. Closed-face filter cassette (CFC) sampling-guidance on
procedures for inclusion of material adhering to internal sampler surfaces. J Occup Environ
Hyg 10(3):D29-33.
ASHRAE [2000]. D-86815 Guideline 12-2000 -- Minimizing the risk of legionellosis
associated with building water systems. Atlanta, GA: American Society of Heating,
Refrigerating and Air-Conditioning Engineers.
ASHRAE [2009]. Indoor air quality guide: best practices for design, construction and
commissioning. Atlanta, GA: American Society of Heating, Refrigerating and Air-
conditioning Engineers.
ASHRAE [2015]. ANSI/ASHRAE Standard 188-2015 Legionellosis: risk management for
building water systems. Atlanta, GA: American Society of Heating, Refrigerating and Air-
Conditioning Engineers.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-68 of BA-115
Sampling and Characterization of Bioaerosols
ASTM [2009]. D7391-09 Standard test method for categorization and quantification of
airborne fungal structures in an inertial impaction sample by optical microscopy. West
Conshohocken, PA: ASTM International.
ASTM [2012]. D7789-12 Standard practice for collection of fungal material from surfaces by
swab. West Conshohocken, PA: ASTM International.
ASTM [2014a]. E1370-14 Standard guide for air sampling strategies for worker and workplace
protection. West Conshohocken, PA: ASTM International.
ASTM [2014b]. D7338-14 Standard guide for assessment of fungal growth in buildings. West
Conshohocken, PA: ASTM International.
ASTM [2014c]. D7910-14 Standard practice for collection of fungal material from surfaces by
tape lift. West Conshohocken, PA: ASTM International.
ASTM [2014d]. D7788-14 Standard practice for collection of total airborne fungal structures
via inertial impaction methodology. West Conshohocken, PA: ASTM International.
ASTM [2014e]. D7297-14 Standard practice for evaluating residential indoor air quality
concerns. West Conshohocken, PA: ASTM International.
ASTM [2015]. ASTM D5952 - 08(2015) Standard guide for the inspection of water systems for
Legionella and the investigation of possible outbreaks of legionellosis (Legionnaires' Disease
or Pontiac Fever). West Conshohocken, PA: ASTM International.
ATS [1990]. American Thoracic Society. Diagnostic standards and classification of
tuberculosis. Am Rev Respir Dis 142(3):725-735.
Axelsson BO, Saraf A, Larsson L [1995]. Determination of ergosterol in organic dust by gas
chromatography-mass spectrometry. J Chromatogr B Biomed Sci Appl 666(1):77-84.
Babady NE, Buckwalter SP, Hall L, Le Febre KM, Binnicker MJ, Wengenack NL [2011].
Detection of Blastomyces dermatitidis and Histoplasma capsulatum from culture isolates and
clinical specimens by use of real-time PCR. J Clin Microbiol 49(9):3204-3208.
Banaszak EF, Thiede WH, Fink JN [1970]. Hypersensitivity pneumonitis due to
contamination of an air conditioner. N Engl J Med 283(6):271-276.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-69 of BA-115
Sampling and Characterization of Bioaerosols
Baron EJ, Finegold SM [1990]. Bailey & Scott's Diagnostic Microbiology, 8
th
edition. St. Louis:
Mosby.
Baron PA, Deye GJ [1990]. Electrostatic effects in asbestos sampling. I: Experimental
measurements. Am Ind Hyg Assoc J 51(2):51-62.
Beaulieu HJ, Fidino AV, Arlington KL, Buchan RM [1980]. A comparison of aerosol sampling
techniques: "open" versus "closed-face" filter cassettes. Am Ind Hyg Assoc J 41(10):758-765.
Bennett JW, Klich M [2003]. Mycotoxins. Clin Microbiol Rev 16(3):497-516.
Bhangar S, Adams RI, Pasut W, Huffman JA, Arens EA, Taylor JW, Bruns TD, Nazaroff WW
[2016]. Chamber bioaerosol study: human emissions of size-resolved fluorescent biological
aerosol particles. Indoor Air 26(2):193-206.
Blachere FM, Cao G, Lindsley WG, Noti JD, Beezhold DH [2011]. Enhanced detection of
infectious airborne influenza virus. J Virol Methods 176(1-2):120-124.
Blachere FM, Lindsley WG, Pearce TA, Anderson SE, Fisher M, Khakoo R, Meade BJ, Lander
O, Davis S, Thewlis RE, Celik I, Chen BT, Beezhold DH [2009]. Measurement of airborne
influenza virus in a hospital emergency department. Clin Infect Dis 48(4):438-440.
Blais Lecours P, Veillette M, Marsolais D, Duchaine C [2012]. Characterization of bioaerosols
from dairy barns: reconstructing the puzzle of occupational respiratory diseases by using
molecular approaches. Appl Environ Microbiol 78(9):3242-3248.
Bolaños-Rosero B, Betancourt D, Dean T, Vesper S [2013]. Pilot study of mold populations
inside and outside of Puerto Rican residences. Aerobiologia 29(4):537-543.
Bonifait L, Charlebois R, Vimont A, Turgeon N, Veillette M, Longtin Y, Jean J, Duchaine C
[2015]. Detection and quantification of airborne norovirus during outbreaks in healthcare
facilities. Clin Infect Dis 61(3):299-304.
Booth TF, Kournikakis B, Bastien N, Ho J, Kobasa D, Stadnyk L, Li Y, Spence M, Paton S,
Henry B, Mederski B, White D, Low DE, McGeer A, Simor A, Vearncombe M, Downey J,
Jamieson FB, Tang P, Plummer F [2005]. Detection of airborne severe acute respiratory
syndrome (SARS) coronavirus and environmental contamination in SARS outbreak units. J
Infect Dis 191(9):1472-1477.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-70 of BA-115
Sampling and Characterization of Bioaerosols
Brasel TL, Douglas DR, Wilson SC, Straus DC [2005]. Detection of airborne Stachybotrys
chartarum macrocyclic trichothecene mycotoxins on particulates smaller than conidia. Appl
Environ Microbiol 71(1):114-122.
Breuer D [2012]. Flow resistance of samplers for personal monitoring in work areas and
requirements for sampling pump performance. J Occup Environ Hyg 9(2):D25-32.
Brockmann JE [2011]. Aerosol transport in sampling lines and inlets. In: Kulkarni P, Baron
PA, Willeke K, eds. Aerosol measurement: principles, techniques, and applications. Hoboken,
NJ: John Wiley & Sons.
Brown RC, Hemingway MA, Wake D, Thorpe A [1996]. Electret-based passive dust sampler:
sampling of organic dusts. Analyst 121(9):1241-1246.
Buiarelli F, Canepari S, Di Filippo P, Perrino C, Pomata D, Riccardi C, Speziale R [2013].
Extraction and analysis of fungal spore biomarkers in atmospheric bioaerosol by HPLC–MS–
MS and GC–MS. Talanta 105:142-151.
Burge HP, Boise JR, Rutherford JA, Solomon WR [1977]. Comparative recoveries of airborne
fungus spores by viable and non-viable modes of volumetric collection. Mycopathologia
61(1):27-33.
Buttner MP, Stetzenbach LD [1993]. Monitoring airborne fungal spores in an experimental
indoor environment to evaluate sampling methods and the effects of human activity on air
sampling. Appl Environ Microbiol 59(1):219-226.
Carvalho E, Sindt C, Verdier A, Galan C, O'Donoghue L, Parks S, Thibaudon M [2008].
Performance of the Coriolis air sampler, a high-volume aerosol-collection system for
quantification of airborne spores and pollen grains. Aerobiologia 24(4):191-201.
Castillo NI, Ibanez M, Beltran E, Rivera-Monroy J, Ochoa JC, Paez-Castillo M, Posada-
Buitrago ML, Sulyok M, Hernandez F [2016]. Identification of mycotoxins by UHPLC-QTOF
MS in airborne fungi and fungi isolated from industrial paper and antique documents from
the Archive of Bogota. Environ Res 144(Pt A):130-138.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-71 of BA-115
Sampling and Characterization of Bioaerosols
CDC [2003]. Guidelines for environmental infection control in health-care facilities.
Recommendations of CDC and the Healthcare Infection Control Practices Advisory
Committee (HICPAC). Atlanta, GA: U.S. Department of Health and Human Services, Centers
for Disease Control and Prevention,
http://www.cdc.gov/hicpac/pdf/guidelines/eic_in_HCF_03.pdf.
CDC [2009]. Biosafety in microbiological and biomedical laboratories, 5
th
edition. Chosewood
LC, Wilson DE, eds. Washington DC: U.S. Department of Health and Human Services,
Centers for Disease Control and Prevention, National Institutes of Health, DHHS (CDC)
Publication No. 21-1112, http://www.cdc.gov/biosafety/publications/bmbl5/index.htm.
CDC [2015]. Sampling procedure and potential sampling sites. Protocol for collecting
environmental samples for Legionella culture during a cluster or outbreak investigation or
when cases of disease may be associated with a facility. Atlanta, GA: U.S. Department of
Health and Human Services, Centers for Disease Control and Prevention,
http://www.cdc.gov/legionella/downloads/cdc-sampling-procedure.pdf.
Cella LN, Blackstock D, Yates MA, Mulchandani A, Chen W [2013]. Detection of RNA
viruses: current technologies and future perspectives. Crit Rev Eukaryot Gene Expr 23(2):125-
137.
CEN [2000]. EN 13098:2000 Workplace atmosphere - Guidelines for measurement of
airborne micro-organisms and endotoxin. Brussels, Belgium: European Committee for
Standardization.
CEN [2003]. EN 14031:2003 Workplace atmospheres - Determination of airborne endotoxins.
Brussels, Belgium: European Committee for Standardization.
CEN [2004]. EN 14583:2004 Workplace atmospheres - Volumetric bioaerosol sampling
devices - Requirements and test methods. Brussels, Belgium: European Committee for
Standardization.
Chang C-W, Chou F-C [2011]. Assessment of bioaerosol sampling techniques for viable
Legionella pneumophila by ethidium monoazide quantitative PCR. Aerosol Sci Technol
45(3):343-351.
Cherrie JW, Maccalman L, Fransman W, Tielemans E, Tischer M, Van Tongeren M [2011].
Revisiting the effect of room size and general ventilation on the relationship between near-
and far-field air concentrations. Ann Occup Hyg 55(9):1006-1015.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-72 of BA-115
Sampling and Characterization of Bioaerosols
Chew GL, Douwes J, Doekes G, Higgins KM, van Strien R, Spithoven J, Brunekreef B [2001].
Fungal extracellular polysaccharides, beta (1 -> 3)-glucans and culturable fungi in repeated
sampling of house dust. Indoor Air 11(3):171-178.
Condit RC [2007]. Principles of virology. In: Knipe DM, Howley PM, eds. Fields virology.
Philadelphia: Lippincott, Williams & Wilkins.
Coons AH, Creech HJ, Jones RN [1941]. Immunological properties of an antigen containing a
fluorescent group. Proc Soc Exp Biol Med 47:200-202.
Coons AH, Creech HJ, Jones RN, Berliner E [1942]. The demonstration of pneumococcal
antigen in tissues by use of fluorescent antibody. J Immunol 45:159-170.
Corzo CA, Culhane M, Dee S, Morrison RB, Torremorell M [2013]. Airborne detection and
quantification of swine influenza a virus in air samples collected inside, outside and
downwind from swine barns. PLoS ONE 8(8):e71444.
Cown WB, Kethley TW, Fincher EL [1957]. The critical-orifice liquid impinger as a sampler
for bacterial aerosols. Appl Microbiol 5(2):119-124.
Cox CS [1968]. The aerosol survival of Escherichia coli B in nitrogen, argon and helium
atmospheres and the influence of relative humidity. J Gen Microbiol 50(1):139-147.
Cox CS [1987]. The aerobiological pathway of microorganisms. New York: Wiley.
Cox CS [1989]. Airborne bacteria and viruses. Sci Prog 73(292 Pt 4):469-499.
Custis NJ, Woodfolk JA, Vaughan JW, Platts-Mills TA [2003]. Quantitative measurement of
airborne allergens from dust mites, dogs, and cats using an ion-charging device. Clin Exp
Allergy 33(7):986-991.
Das R, McNary J, Fitzsimmons K, Dobraca D, Cummings K, Mohle-Boetani J, Wheeler C,
McDowell A, Iossifova Y, Bailey R, Kreiss K, Materna B [2012]. Occupational
coccidioidomycosis in California: outbreak investigation, respirator recommendations, and
surveillance findings. J Occup Environ Med 54(5):564-571.
Davies A, Thomson G, Walker J, Bennett A [2009]. A review of the risks and disease
transmission associated with aerosol generating medical procedures. J Infect Prev 10(4):122-
126.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-73 of BA-115
Sampling and Characterization of Bioaerosols
Demirjian A, Lucas CE, Garrison LE, Kozak-Muiznieks NA, States S, Brown EW, Wortham
JM, Beaudoin A, Casey ML, Marriott C, Ludwig AM, Sonel AF, Muder RR, Hicks LA [2015].
The importance of clinical surveillance in detecting Legionnaires' disease outbreaks: a large
outbreak in a hospital with a Legionella disinfection system-Pennsylvania, 2011-2012. Clin
Infect Dis 60(11):1596-1602.
Dennis PJL [1990]. An unnecessary risk: Legionnaires' disease. In: Morey PR, Feeley JC, Otten
JA, eds. Biological contaminants in indoor environments. Philadelphia: ASTM.
Donaldson AI, Ferris NP, Gloster J [1982]. Air sampling of pigs infected with foot-and-mouth
disease virus: comparison of Litton and cyclone samplers. Res Vet Sci 33(3):384-385.
Douwes J, Doekes G, Montijn R, Heederik D, Brunekreef B [1997]. An immunoassay for the
measurement of (1 -> 3)-beta-D-glucans in the indoor environment. Mediators Inflamm
6(4):257-262.
Dungan RS, Leytem AB [2015]. Recovery of culturable Escherichia coli O157:H7 during
operation of a liquid-based bioaerosol sampler. Aerosol Sci Technol 50(1):71-75.
Dyer RL, Frank JF, Johnson B, Hickey P, Fitts J [2004]. Microbiological tests for equipment,
containers, water, and air. In: Wehr HM, Frank JF, eds. Standard methods for the examination
of dairy products. Washington, DC: American Public Health Association.
Eduard W [2009]. Fungal spores: a critical review of the toxicological and epidemiological
evidence as a basis for occupational exposure limit setting. Crit Rev Toxicol 39(10):799-864.
Eduard W, Heederik D, Duchaine C, Green BJ [2012]. Bioaerosol exposure assessment in the
workplace: the past, present and recent advances. J Environ Monit 14(2):334-339.
Eduard W, Lacey J, Karlsson K, Palmgren U, Strom G, Blomquist G [1990]. Evaluation of
methods for enumerating microorganisms in filter samples from highly contaminated
occupational environments. Am Ind Hyg Assoc J 51(8):427-436.
Environment Agency [2009]. Review of methods to measure bioaerosols from composting
sites. Science report: SC040021/SR3. Bristol, UK: Environment Agency.
Fabian P, McDevitt JJ, Houseman EA, Milton DK [2009]. Airborne influenza virus detection
with four aerosol samplers using molecular and infectivity assays: considerations for a new
infectious virus aerosol sampler. Indoor Air 19(5):433-441.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-74 of BA-115
Sampling and Characterization of Bioaerosols
Farnsworth JE, Goyal SM, Kim SW, Kuehn TH, Raynor PC, Ramakrishnan MA,
Anantharaman S, Tang W [2006]. Development of a method for bacteria and virus recovery
from heating, ventilation, and air conditioning (HVAC) filters. J Environ Monit 8(10):1006-
1013.
Ferro AR, Kopperud RJ, Hildemann LM [2004]. Source strengths for indoor human activities
that resuspend particulate matter. Environ Sci Technol 38(6):1759-1764.
Fields BN, Knipe DM, Howley PM [2007]. Fields virology. Philadelphia: Lippincott, Williams
& Wilkins.
Fields BS [2002]. Legionellae and Legionnaires’ disease. In: Hurst CJ, Crawford RL,
McInerney MJ, Knudsen GR, Stetzenbach LD, eds. Manual of environmental microbiology.
Washington, DC: ASM Press.
Filep S, Tsay A, Vailes L, Gadermaier G, Ferreira F, Matsui E, King EM, Chapman MD [2012].
A multi-allergen standard for the calibration of immunoassays: CREATE principles applied to
eight purified allergens. Allergy 67(2):235-241.
Fisher EM, Richardson AW, Harpest SD, Hofacre KC, Shaffer RE [2012]. Reaerosolization of
MS2 bacteriophage from an N95 filtering facepiece respirator by simulated coughing. Ann
Occup Hyg 56(3):315-325.
Flannigan B, Samson RA, Miller JD [2011]. Microorganisms in home and indoor work
environments: diversity, health impacts, investigation and control, 2
nd
Edition. Boca Raton,
FL: CRC Press, Taylor and Francis Group.
Flint SJ, Enquist LW, Racaniello VR, Skalka AM [2009a]. Principles of virology, 3
rd
edition.
Washington, DC: American Society for Microbiology.
Flint JS, Enquist LW, Shalka AM [2009b]. Virological methods. In: Principles of virology, 3rd
edition. Washington, DC: American Society for Microbiology.
Frankel M, Timm M, Hansen EW, Madsen AM [2012]. Comparison of sampling methods for
the assessment of indoor microbial exposure. Indoor Air 22(5):405-414.
Frenz DA [1999]. Comparing pollen and spore counts collected with the Rotorod Sampler and
Burkard spore trap. Ann Allergy Asthma Immunol 83(5):341-347; quiz 348-349.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-75 of BA-115
Sampling and Characterization of Bioaerosols
Friberg B, Friberg S, Burman LG [1999]. Inconsistent correlation between aerobic bacterial
surface and air counts in operating rooms with ultra clean laminar air flows: proposal of a new
bacteriological standard for surface contamination. J Hosp Infect 42(4):287-293.
Fronczek CF, Yoon JY [2015]. Biosensors for monitoring airborne pathogens. J Lab Autom
20(4):390-410.
Garvey JS, Campbell DH, Cremer NE, Sussdorf DH [1977]. Methods in immunology: a
laboratory text for instruction and research. Reading, MA: W. A. Benjamin.
Gordon J, Gandhi P, Shekhawat G, Frazier A, Hampton-Marcell J, Gilbert JA [2015]. A simple
novel device for air sampling by electrokinetic capture. Microbiome 3:79.
Gore RB, Hadi EA, Craven M, Smillie FI, O'Meara TJ, Tovey ER, Woodcock A, Custovic A
[2002]. Personal exposure to house dust mite allergen in bed: nasal air sampling and reservoir
allergen levels. Clin Exp Allergy 32(6):856-859.
Görner P, Fabriès JF, Duquenne P, Witschger O, Wrobel R [2006]. Bioaerosol sampling by a
personal rotating cup sampler CIP 10-M. J Environ Monit 8(1):43-48.
Gorny RL, Dutkiewicz J, Krysinska-Traczyk E [1999]. Size distribution of bacterial and fungal
bioaerosols in indoor air. Ann Agric Environ Med 6(2):105-113.
Goyal SM, Anantharaman S, Ramakrishnan MA, Sajja S, Kim SW, Stanley NJ, Farnsworth JE,
Kuehn TH, Raynor PC [2011]. Detection of viruses in used ventilation filters from two large
public buildings. Am J Infect Control 39(7):e30-38.
Graham JA, Pavlicek PK, Sercombe JK, Xavier ML, Tovey ER [2000]. The nasal air sampler: a
device for sampling inhaled aeroallergens. Ann Allergy Asthma Immunol 84(6):599-604.
Gralton J, Tovey ER, McLaws ML, Rawlinson WD [2013]. Respiratory virus RNA is detectable
in airborne and droplet particles. J Med Virol 85(12):2151-2159.
Green BJ, Millechia L, Blachere FM, Tovey ER, Beezhold DH, Schmechel D [2006a]. Dual
fluorescent halogen immunoassay for bioaerosols using confocal microscopy. Anal Biochem
354(1):151-153.
Green BJ, Mitakakis TZ, Tovey ER [2003]. Allergen detection from 11 fungal species before
and after germination. J Allergy Clin Immunol 111(2):285-289.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-76 of BA-115
Sampling and Characterization of Bioaerosols
Green BJ, Schmechel D, Sercombe JK, Tovey ER [2005a]. Enumeration and detection of
aerosolized Aspergillus fumigatus and Penicillium chrysogenum conidia and hyphae using a
novel double immunostaining technique. J Immunol Methods 307(1-2):127-134.
Green BJ, Schmechel D, Summerbell RC [2011]. Aerosolized fungal fragments. In: Adnan O,
Samson RA, eds. Fundamentals of mold growth in indoor environments and strategies for
healthy living. Amsterdam, Netherlands: Wageningen Academic Publishers.
Green BJ, Schmechel D, Tovey ER [2005b]. Detection of Alternaria alternata conidia and
hyphae using a novel double immunostaining technique. Clin Diagn Lab Immunol
12(9):1114-1116.
Green BJ, Sercombe JK, Tovey ER [2005c]. Fungal fragments and undocumented conidia
function as new aeroallergen sources. J Allergy Clin Immunol 115(5):1043-1048.
Green BJ, Tovey ER, Sercombe JK, Blachere FM, Beezhold DH, Schmechel D [2006b].
Airborne fungal fragments and allergenicity. Med Mycol 44:S245-S255.
Green BJ, Yli-Panula E, Tovey ER [2006c]. Halogen immunoassay, a new method for the
detection of sensitization to fungal allergens; comparisons with conventional techniques.
Allergol Int 55(2):131-139.
Greenberg AE, Clesceri LS, Eaton AD [1992]. Standard methods for the examination of water
and wastewater. Washington, DC: American Public Health Association.
Greenburg L [1932]. The Greenburg-Smith impinger sampling apparatus for dusts, fumes,
smoke, and gases. Am J Public Health 22(10):1077-1082.
Grinshpun SA, Willeke K, Ulevicius V, Juozaitis A, Terzieva S, Donnelly J, Stelma GN,
Brenner KP [1997]. Effect of impaction, bounce and reaerosolization on the collection
efficiency of impingers. Aerosol Sci Technol 26(4):326-342.
Gustin KM, Belser JA, Wadford DA, Pearce MB, Katz JM, Tumpey TM, Maines TR [2011].
Influenza virus aerosol exposure and analytical system for ferrets. Proc Natl Acad Sci USA
108(20):8432-8437.
Haaland D, Siegel JA [2016]. Quantitative filter forensics for indoor particle sampling. Indoor
Air, DOI:10.1111/ina.12319.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-77 of BA-115
Sampling and Characterization of Bioaerosols
Haghani I, Shokohi T, Hajheidari Z, Khalilian A, Aghili SR [2013]. Comparison of diagnostic
methods in the evaluation of onychomycosis. Mycopathologia 175(3-4):315-321.
Haig CW, Mackay WG, Walker JT, Williams C [2016]. Bioaerosol sampling: sampling
mechanisms, bioefficiency and field studies. J Hosp Infect 95:242-245
Hairston PP, Ho J, Quant FR [1997]. Design of an instrument for real-time detection of
bioaerosols using simultaneous measurement of particle aerodynamic size and intrinsic
fluorescence. J Aerosol Sci 28(3):471-482.
Han T, Fennell D, Mainelis G [2015]. Development and optimization of the electrostatic
precipitator with superhydrophobic surface (EPSS) Mark II for collection of bioaerosols.
Aerosol Sci Technol 49(4):210-219.
Han T, Mainelis G [2012]. Investigation of inherent and latent internal losses in liquid-based
bioaerosol samplers. J Aerosol Sci 45:58-68.
Harper GJ [1961]. Airborne micro-organisms: survival tests with four viruses. J Hyg (Lond)
59:479-486.
Hatch MT, Warren JC [1969]. Enhanced recovery of airborne T3 coliphage and Pasteurella
pestis bacteriophage by means of a presampling humidification technique. Appl Microbiol
17(5):685-689.
Heederik D [2013]. Potential public health consequences of exposure assessment for
Staphylococcus aureus: commentary on the paper by Masclaux et al. Ann Occup Hyg
57(5):545-549.
Heitkamp MA, McKinsey PC, Bagwell CE, Eakle R, Lindler LE, Fernandez-Prada C [2006].
Feasibility of the Aerosol-to-Liquid Particle Extraction System (ALPES) for collection of
viable Francisella sp. Savannah, GA: Savannah River National Laboratory,
http://sti.srs.gov/fulltext/2006/TR2006281.pdf.
Henningson EW, Ahlberg MS [1994]. Evaluation of microbiological aerosol samplers. A
review. J Aerosol Sci 25(8):1459-1459.
Henningson EW, Fängmark I, Larsson E, Wikström LE [1988]. Collection efficiency of liquid
samplers for microbiological aerosols. J Aerosol Sci 19(7):911-914.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-78 of BA-115
Sampling and Characterization of Bioaerosols
Hering SV [2001]. Impactors, cyclones, and other particle collectors. In: Cohen BS,
McCammon CS Jr., eds. Air sampling instruments for evaluation of atmospheric
contaminants. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
Hinds WC [1999]. Aerosol technology. Properties, behavior, and measurement of airborne
particles. New York: John Wiley & Sons.
Hirst JM [1952]. An automatic volumetric spore-trap. Ann Appl Biol 39:257-265.
Ho J, Spence M, Duncan S [2005]. An approach towards characterizing a reference sampler
for culturable biological particle measurement. J Aerosol Sci 36(5-6):557-573.
Hocking AD, Pitt JI [1980]. Dichloran-glycerol medium for enumeration of xerophilic fungi
from low-moisture foods. Appl Environ Microbiol 39(3):488-492.
Hodges LR, Rose LJ, O'Connell H, Arduino MJ [2010]. National validation study of a swab
protocol for the recovery of Bacillus anthracis spores from surfaces. J Microbiol Methods
81(2):141-146.
Hodges LR, Rose LJ, Peterson A, Noble-Wang J, Arduino MJ [2006]. Evaluation of a
macrofoam swab protocol for the recovery of Bacillus anthracis spores from a steel surface.
Appl Environ Microbiol 72(6):4429-4430.
Hogan CJ Jr., Kettleson EM, Lee MH, Ramaswami B, Angenent LT, Biswas P [2005]. Sampling
methodologies and dosage assessment techniques for submicrometre and ultrafine virus
aerosol particles. J Appl Microbiol 99(6):1422-1434.
Holmgren H, Bake B, Olin AC, Ljungstrom E [2011]. Relation between humidity and size of
exhaled particles. J Aerosol Med Pulm Drug Deliv 24(5):253-260.
Hook-Barnard I, Norris SMP, Alper J [2014]. Technologies to enable autonomous detection
for BioWatch: ensuring timely and accurate information for public health officials: workshop
summary. Washington DC: Institute of Medicine.
HUD [2008]. Vacuum dust sample collection protocol for allergens. Washington, DC:
Department of Housing and Urban Development,
http://portal.hud.gov/hudportal/documents/huddoc?id=DOC_12539.pdf.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-79 of BA-115
Sampling and Characterization of Bioaerosols
Hung LL, Miller JD, Dillon HK [2005]. Field guide for the determination of biological
contaminants in environmental samples. Fairfax, VA: American Industrial Hygiene
Association.
Hunter CA, Grant C, Flannigan B, Bravery AF [1988]. Mould in buildings: the air spora of
domestic dwellings. Int Biodeterior 24(2):81-101.
Hussein T, Norros V, Hakala J, Petaja T, Aalto PP, Rannik U, Vesala T, Ovaskainen O [2013].
Species traits and inertial deposition of fungal spores. J Aerosol Sci 61(0):81-98.
Huttunen K, Tirkkonen J, Taubel M, Krop E, Mikkonen S, Pekkanen J, Heederik D, Zock JP,
Hyvarinen A, Hirvonen MR [2016]. Inflammatory potential in relation to the microbial
content of settled dust samples collected from moisture-damaged and reference schools:
results of HITEA study. Indoor Air 26(3):380-390.
Hyvarinen A, Vahteristo M, Meklin T, Jantunen M, Nevalainen A, Moschandreas D [2001].
Temporal and spatial variation of fungal concentrations in indoor air. Aerosol Sci Technol
35(2):688-695.
Ibach MJ, Larsh HW, Furcolow ML [1954]. Isolation of Histoplasma capsulatum from the air.
Science 119(3080):71.
Ijaz MK, Brunner AH, Sattar SA, Nair RC, Johnson-Lussenburg CM [1985a]. Survival
characteristics of airborne human coronavirus 229E. J Gen Virol 66(Pt 12):2743-2748.
Ijaz MK, Sattar SA, Johnson-Lussenburg CM, Springthorpe VS, Nair RC [1985b]. Effect of
relative humidity, atmospheric temperature, and suspending medium on the airborne survival
of human rotavirus. Can J Microbiol 31(8):681-685.
Ijaz MK, Zargar B, Wright KE, Rubino JR, Sattar SA [2016]. Generic aspects of the airborne
spread of human pathogens indoors and emerging air decontamination technologies. Am J
Infect Control 44(9 Suppl):S109-120.
IOM [2004]. Damp indoor spaces and health. Washington, DC: Institute of Medicine.
ISO [2003]. ISO 14698:2003 Cleanrooms and associated controlled environments --
Biocontamination control. Geneva, Switzerland: International Organization for
Standardization.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-80 of BA-115
Sampling and Characterization of Bioaerosols
ISO [2012]. ISO 13138:2012 Air quality -- Sampling conventions for airborne particle
deposition in the human respiratory system. Geneva, Switzerland: International Organization
for Standardization.
Jacobs RR [1989]. Airborne endotoxins: an association with occupational lung disease. Appl
Ind Hyg 4(2):50-56.
Jensen PA, Todd WF, Davis GN, Scarpino PV [1992]. Evaluation of eight bioaerosol samplers
challenged with aerosols of free bacteria. Am Ind Hyg Assoc J 53(10):660-667.
Johansson E, Reponen T, Meller J, Vesper S, Yadav J [2014]. Association of Streptomyces
community composition determined by PCR-denaturing gradient gel electrophoresis with
indoor mold status. Environ Monit Assess 186(12):8773-8783.
Johnson FB [1990]. Transport of viral specimens. Clin Microbiol Rev 3(2):120-131.
Johnston AM, Vincent JH, Jones AD [1985]. Measurements of electric charge for workplace
aerosols. Ann Occup Hyg 29(2):271-284.
Jones RM, Brosseau LM [2015]. Aerosol transmission of infectious disease. J Occup Environ
Med 57(5):501-508.
Jonges M, van Leuken J, Wouters I, Koch G, Meijer A, Koopmans M [2015]. Wind-mediated
spread of low-pathogenic avian influenza virus into the environment during outbreaks at
commercial poultry farms. PLoS ONE 10(5):e0125401.
Julian TR, Tamayo FJ, Leckie JO, Boehm AB [2011]. Comparison of surface sampling
methods for virus recovery from fomites. Appl Environ Microbiol 77(19):6918-6925.
Kanaani H, Hargreaves M, Smith J, Ristovski Z, Agranovski V, Morawska L [2008].
Performance of UVAPS with respect to detection of airborne fungi. J Aerosol Sci 39(2):175-
189.
Karim YG, Ijaz MK, Sattar SA, Johnson-Lussenburg CM [1985]. Effect of relative humidity on
the airborne survival of rhinovirus-14. Can J Microbiol 31(11):1058-1061.
Karlsson K, Malmberg P [1989]. Characterization of exposure to molds and actinomycetes in
agricultural dusts by scanning electron microscopy, fluorescence microscopy and the culture
method. Scand J Work Environ Health 15(5):353-359.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-81 of BA-115
Sampling and Characterization of Bioaerosols
Kendrick B [2000]. The fifth kingdom, 3
rd
Edition. Newburyport, MA: Focus Publishing, R.
Pullins Company.
Kenny LC, Aitken R, Chalmers C, Fabries JF, Gonzalez-Fernandez E, Kromhout H, Liden G,
Mark D, Riediger G, Prodi V [1997]. A collaborative European study of personal inhalable
aerosol sampler performance. Ann Occup Hyg 41(2):135-153.
Kesavan J, Sagripanti JL [2015]. Evaluation criteria for bioaerosol samplers. Environ Sci
Process Impacts 17(3):638-645.
Kesavan J, Schepers D, McFarland AR [2010]. Sampling and retention efficiencies of batch-
type liquid-based bioaerosol samplers. Aerosol Sci Technol 44(10):817-829.
Kesavan JS, Schepers D, Bottiger J [2011]. Characteristics of twenty-nine aerosol samplers
tested at U.S. Army Edgewood Chemical Biological Center (2000 - 2006). Aberdeen Proving
Ground, MD: U.S. Army, http://www.dtic.mil/dtic/tr/fulltext/u2/a540009.pdf.
Kettleson E, Kumar S, Reponen T, Vesper S, Meheust D, Grinshpun SA, Adhikari A [2013].
Stenotrophomonas, Mycobacterium, and Streptomyces in home dust and air: associations
with moldiness and other home/family characteristics. Indoor Air 23(5):387-396.
Kettleson EM, Adhikari A, Vesper S, Coombs K, Indugula R, Reponen T [2015]. Key
determinants of the fungal and bacterial microbiomes in homes. Environ Res 138:130-135.
Kilburg-Basnyat B, Metwali N, Thorne PS [2016]. Performance of electrostatic dust collectors
(EDCs) for endotoxin assessment in homes: effect of mailing, placement, heating, and
electrostatic charge. J Occup Environ Hyg 13(2):85-93.
Kilburg-Basnyat B, Peters TM, Perry SS, Thorne PS [2015]. Electrostatic dust collectors
compared to inhalable samplers for measuring endotoxin concentrations in farm homes.
Indoor Air 26(5):724-733.
King EM, Filep S, Smith B, Platts-Mills T, Hamilton RG, Schmechel D, Sordillo JE, Milton D,
van Ree R, Krop EJ, Heederik DJ, Metwali N, Thorne PS, Zeldin DC, Sever ML, Calatroni A,
Arbes SJ Jr., Mitchell HE, Chapman MD [2013]. A multi-center ring trial of allergen analysis
using fluorescent multiplex array technology. J Immunol Methods 387(1-2):89-95.
Kissell FN, Sacks HK [2002]. Inaccuracy of area sampling for measuring the dust exposure of
mining machine operators in coal mines. Mining Eng 54(2):33-39.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-82 of BA-115
Sampling and Characterization of Bioaerosols
Koncsag CI, Barbulescu A [2011]. Liquid-liquid extraction with and without a chemical
reaction. In: El-Amin M, ed. Mass transfer in multiphase systems and its applications. Rijeka,
Croatia: InTech. DOI: 10.5772/594.
Koneman EW [1988]. Color atlas and textbook of diagnostic microbiology. Philadelphia:
Lippincott.
Kujundzic E, Hernandez M, Miller SL [2006]. Particle size distributions and concentrations of
airborne endotoxin using novel collection methods in homes during the winter and summer
seasons. Indoor Air 16(3):216-226.
Kuo Y-M [2015]. Field evaluation of sampling bias with plastic petri dishes for size-
fractionated bioaerosol sampling. Aerosol Sci Technol 49(3):127-133.
Lacey J, Crook B [1988]. Fungal and actinomycete spores as pollutants of the workplace and
occupational allergens. Ann Occup Hyg 32(4):515-533.
LeBouf R, Yesse L, Rossner A [2008]. Seasonal and diurnal variability in airborne mold from
an indoor residential environment in Northern New York. J Air Waste Manag Assoc
58(5):684-692.
Lednicky J, Pan M, Loeb J, Hsieh H, Eiguren-Fernandez A, Hering S, Fan ZH, Wu C-Y [2016].
Highly efficient collection of infectious pandemic influenza H1N1 virus (2009) through
laminar-flow water based condensation. Aerosol Sci Technol 50(7):i-iv.
Lee KS, Bartlett KH, Brauer M, Stephens GM, Black WA, Teschke K [2004a]. A field
comparison of four samplers for enumerating fungal aerosols I. Sampling characteristics.
Indoor Air 14(5):360-366.
Lee SA, Adhikari A, Grinshpun SA, McKay R, Shukla R, Reponen T [2006]. Personal exposure
to airborne dust and microorganisms in agricultural environments. J Occup Environ Hyg
3(3):118-130.
Lee SA, Liao CH [2014]. Size-selective assessment of agricultural workers' personal exposure
to airborne fungi and fungal fragments. Sci Total Environ 466-467:725-732.
Lee SA, Willeke K, Mainelis G, Adhikari A, Wang H, Reponen T, Grinshpun SA [2004b].
Assessment of electrical charge on airborne microorganisms by a new bioaerosol sampling
method. J Occup Environ Hyg 1(3):127-138.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-83 of BA-115
Sampling and Characterization of Bioaerosols
Leland DS, Emanuel D [1995]. Laboratory diagnosis of viral infections of the lung. Semin
Respir Infect 10(4):189-198.
Leland DS, Ginocchio CC [2007]. Role of cell culture for virus detection in the age of
technology. Clin Microbiol Rev 20(1):49-78.
Lembke LL, Kniseley RN, van Nostrand RC, Hale MD [1981]. Precision of the all-glass
impinger and the Andersen microbial impactor for air sampling in solid-waste handling
facilities. Appl Environ Microbiol 42(2):222-225.
Leopold SS [1988]. "Positive hole" statistical adjustment for a two-stage, 200-hole-per-stage
Andersen air sampler. Am Ind Hyg Assoc J 49(2):A88-A90.
Life Technologies [2014]. Real-time PCR handbook. Waltham, MA: Thermo Fisher Scientific,
http://www.thermofisher.com/us/en/home/life-science/pcr/real-time-pcr/qpcr-
education.html.
Lin X, Willeke K, Ulevicius V, Grinshpun SA [1997]. Effect of sampling time on the collection
efficiency of all-glass impingers. Am Ind Hyg Assoc J 58(7):480-488.
Lin XJ, Reponen T, Willeke K, Wang Z, Grinshpun SA, Trunov M [2000]. Survival of airborne
microorganisms during swirling aerosol collection. Aerosol Sci Technol 32(3):184-196.
Lindsley WG, Blachere FM, Davis KA, Pearce TA, Fisher MA, Khakoo R, Davis SM, Rogers
ME, Thewlis RE, Posada JA, Redrow JB, Celik IB, Chen BT, Beezhold DH [2010a].
Distribution of airborne influenza virus and respiratory syncytial virus in an urgent care
medical clinic. Clin Infect Dis 50(5):693-698.
Lindsley WG, Blachere FM, Thewlis RE, Vishnu A, Davis KA, Cao G, Palmer JE, Clark KE,
Fisher MA, Khakoo R, Beezhold DH [2010b]. Measurements of airborne influenza virus in
aerosol particles from human coughs. PLoS ONE 5(11):e15100.
Lioy PJ, Freeman NC, Millette JR [2002]. Dust: a metric for use in residential and building
exposure assessment and source characterization. Environ Health Perspect 110(10):969-983.
Liu BYH, Pui DYH, Rubow KL, Szymanski WW [1985]. Electrostatic effects in aerosol
sampling and filtration. Aerosol Sci Technol 4(3):249-268.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-84 of BA-115
Sampling and Characterization of Bioaerosols
Lowen AC, Mubareka S, Steel J, Palese P [2007]. Influenza virus transmission is dependent on
relative humidity and temperature. PLoS Pathog 3(10):1470-1476.
Lowen AC, Steel J, Mubareka S, Palese P [2008]. High temperature (30 degrees C) blocks
aerosol but not contact transmission of influenza virus. J Virol 82(11):5650-5652.
Macher JM [1989]. Positive-hole correction of multiple-jet impactors for collecting viable
microorganisms. Am Ind Hyg Assoc J 50(11):561-568.
Macher JM [1999]. Bioaerosols: assessment and control. Cincinnati, OH: American
Conference of Governmental Industrial Hygienists.
Macher JM, Chatigny MA, Burge HA [1995]. Sampling airborne microorganisms and
aeroallergens. In: Cohen BS, Hering SV, eds. Air sampling instruments for evaluation of
atmospheric contaminants. Cincinnati, OH: American Conference of Governmental
Industrial Hygienists.
Macher JM, First MW [1984]. Personal air samplers for measuring occupational exposures to
biological hazards. Am Ind Hyg Assoc J 45(2):76-83.
Macher JM, Hansson HC [1987]. Personal size-separating impactor for sampling
microbiological aerosols. Am Ind Hyg Assoc J 48(7):652-655.
Madeley CR, Peiris JS [2002]. Methods in virus diagnosis: immunofluorescence revisited. J
Clin Virol 25(2):121-134.
Madelin TM, Johnson HE [1992]. Fungal and actinomycete spore aerosols measured at
different humidities with an aerodynamic particle sizer. J Appl Bacteriol 72(5):400-409.
Mahony JB [2008]. Detection of respiratory viruses by molecular methods. Clin Microbiol Rev
21(4):716-747.
Mainelis G, Gorny RL, Reponen T, Trunov M, Grinshpun SA, Baron P, Yadav J, Willeke K
[2002]. Effect of electrical charges and fields on injury and viability of airborne bacteria.
Biotechnol Bioeng 79(2):229-241.
Marple VA, Olson BA [2011]. Sampling and measurement using inertial, gravitational,
centrifugal, and thermal techniques. In: Kulkarni P, Baron PA, Willeke K, eds. Aerosol
measurement: principles, techniques, and applications. Hoboken, NJ: John Wiley & Sons.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-85 of BA-115
Sampling and Characterization of Bioaerosols
Marple VA, Willeke K [1976]. Inertial impactors: theory, design and use. In: Liu BYH, ed.
Fine particles: aerosol generation, measurement, sampling and analysis. New York: Academic
Press.
Martin E, Dziurowitz N, Jackel U, Schafer J [2015]. Detection of airborne bacteria in a duck
production facility with two different personal air sampling devices for an exposure
assessment. J Occup Environ Hyg 12(2):77-86.
Martyny JW, Martinez KF, Morey PR [1999]. Source Sampling. In: Macher J, ed. Bioaerosols:
assessment and control. Cincinnati, OH: American Conference of Governmental Industrial
Hygienists.
Masclaux FG, Hotz P, Gashi D, Savova-Bianchi D, Oppliger A [2014]. Assessment of airborne
virus contamination in wastewater treatment plants. Environ Res 133:260-265.
May KR [1966]. Multistage liquid impinger. Bacteriol Rev 30(3):559-570.
May KR, Harper GJ [1957]. The efficiency of various liquid impinger samplers in bacterial
aerosols. Br J Ind Med 14(4):287-297.
McDevitt JJ, Koutrakis P, Ferguson ST, Wolfson JM, Fabian MP, Martins M, Pantelic J,
Milton DK [2013]. Development and performance evaluation of an exhaled-breath bioaerosol
collector for influenza virus. Aerosol Sci Technol 47(4):444-451.
Meadow JF, Altrichter AE, Bateman AC, Stenson J, Brown GZ, Green JL, Bohannan BJ [2015].
Humans differ in their personal microbial cloud. PeerJ 3:e1258.
Meklin T, Reponen T, Toivola M, Koponen V, Husman T, Hyvarinen A, Nevalainen A [2002].
Size distributions of airborne microbes in moisture-damaged and reference school buildings
of two construction types. Atmos Environ 36(39-40):6031-6039.
Mendell MJ, Mirer AG, Cheung K, Tong M, Douwes J [2011]. Respiratory and allergic health
effects of dampness, mold, and dampness‑ related agents: a review of the epidemiologic
evidence. Environ Health Perspect 119:748-756.
Mikhailov E, Vlasenko S, Niessner R, Poschl U [2004]. Interaction of aerosol particles
composed of protein and salts with water vapor: hygroscopic growth and microstructural
rearrangement. Atmos Chem Phys 4(2):323-350.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-86 of BA-115
Sampling and Characterization of Bioaerosols
Milton DK, Fabian MP, Cowling BJ, Grantham ML, McDevitt JJ [2013]. Influenza virus
aerosols in human exhaled breath: particle size, culturability, and effect of surgical masks.
PLoS Pathog 9(3):e1003205.
Milton DK, Gere RJ, Feldman HA, Greaves IA [1990]. Endotoxin measurement: aerosol
sampling and application of a new Limulus method. Am Ind Hyg Assoc J 51(6):331-337.
Mitakakis TZ, Barnes C, Tovey ER [2001]. Spore germination increases allergen release from
Alternaria. J Allergy Clin Immunol 107(2):388-390.
Mitakakis TZ, O'Meara TJ, Tovey ER [2003]. The effect of sunlight on allergen release from
spores of the fungus Alternaria. Grana 42(1):43-46.
Mitakakis TZ, Tovey ER, Xuan W, Marks GB [2000]. Personal exposure to allergenic pollen
and mould spores in inland New South Wales, Australia. Clin Exp Allergy 30(12):1733-1739.
Moore G, Griffith C [2007]. Problems associated with traditional hygiene swabbing: the need
for in-house standardization. J Appl Microbiol 103(4):1090-1103.
Morey P, Otten J, Burge H, Chatigny M, Feeley J, LaForce FM, Peterson K [1986]. Airborne
viable microorganisms in office environments: sampling protocol and analytical procedures.
Appl Ind Hyg 1:R19-R23.
Morey PR [2007]. Microbiological sampling strategies in indoor environments. In: Sampling
and analysis of indoor microorganisms. Hoboken, NJ: John Wiley & Sons, Inc.
Murray BK, Ohmine S, Tomer DP, Jensen KJ, Johnson FB, Kirsi JJ, Robison RA, O'Neill KL
[2008]. Virion disruption by ozone-mediated reactive oxygen species. J Virol Methods
153(1):74-77.
Nardell EA [2016]. Indoor environmental control of tuberculosis and other airborne
infections. Indoor Air 26(1):79-87.
Nazaroff WW [2016]. Indoor bioaerosol dynamics. Indoor Air 26(1):61-78.
Nevalainen A [1989]. Bacterial aerosols in indoor air. Thesis. Helsinki, Finland: National
Public Health Institute.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-87 of BA-115
Sampling and Characterization of Bioaerosols
Nevalainen A, Taubel M, Hyvarinen A [2015]. Indoor fungi: companions and contaminants.
Indoor Air 25(2):125-156.
Nicas M, Nazaroff WW, Hubbard A [2005]. Toward understanding the risk of secondary
airborne infection: emission of respirable pathogens. J Occup Environ Hyg 2(3):143-154.
NIOSH [2003a]. Aerosol sampling: minimizing particle loss from cassette bypass leakage. By
Baron PA. In: Schlecht PC, O’Connor PF, eds. NIOSH Manual of Analytical Methods, 4th
edition. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease
Control and Prevention, National Institute for Occupational Safety and Health, DHHS
(NIOSH) Publication No. 2003-154, http://www.cdc.gov/niosh/nmam.
NIOSH [2003b]. Method 0800 Bioaerosol sampling (indoor air). In: Schlecht PC, O’Connor
PF, eds. NIOSH Manual of Analytical Methods, 4th edition. Cincinnati, OH: U.S. Department
of Health and Human Services, Centers for Disease Control and Prevention, National
Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2003-154,
http://www.cdc.gov/niosh/nmam.
NIOSH [2003c]. Method 0600 Particulates not otherwise regulated, respirable. In: Schlecht
PC, O’Connor PF, eds. NIOSH Manual of Analytical Methods, 4th edition. Cincinnati, OH:
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,
National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2003-
154, http://www.cdc.gov/niosh/nmam.
NIOSH [2003d]. Method 2549 Volatile organic compounds (screening). In: Schlecht PC,
O’Connor PF, eds. NIOSH Manual of Analytical Methods, 4th edition. Cincinnati, OH: U.S.
Department of Health and Human Services, Centers for Disease Control and Prevention,
National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2003-
154, http://www.cdc.gov/niosh/nmam.
NIOSH [2003e]. NIOSH Manual of Analytical Methods, 4th edition. Schlecht PC, O’Connor
PF, eds. Cincinnati, OH: U.S. Department of Health and Human Services, Centers for Disease
Control and Prevention, National Institute for Occupational Safety and Health, DHHS
(NIOSH) Publication No. 2003-154, http://www.cdc.gov/niosh/nmam.
NIOSH [2010]. NIOSH Pocket Guide to Chemical Hazards. Cincinnati, OH: U.S. Department
of Health and Human Services, Centers for Disease Control and Prevention, National
Institute for Occupational Safety and Health, http://www.cdc.gov/niosh/npg/.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-88 of BA-115
Sampling and Characterization of Bioaerosols
NIOSH [2012a] Preventing occupational respiratory disease from exposures caused by
dampness in office buildings, schools, and other nonindustrial buildings. Morgantown, WV:
U.S. Department of Health and Human Services, Centers for Disease Control and Prevention,
National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2013-
102, http://www.cdc.gov/niosh/docs/2013-102/pdfs/2013-102.pdf.
NIOSH [2012b]. Surface sampling procedures for Bacillus anthracis spores from smooth,
non-porous surfaces. Washington, D.C.: U.S. Department of Health and Human Services,
Centers for Disease Control and Prevention, National Institute for Occupational Safety and
Health, http://www.cdc.gov/niosh/topics/emres/surface-sampling-bacillus-anthracis.html.
NIOSH [2016a]. Factors affection aerosol sampling. By Baron PA. In: Ashley K, O’Connor PF,
eds. NIOSH Manual of Analytical Methods, 5th edition. Cincinnati, OH: U.S. Department of
Health and Human Services, Centers for Disease Control and Prevention, National Institute
for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2014-151,
http://www.cdc.gov/niosh/nmam.
NIOSH [2016b]. Filter pore size and aerosol sample collection. By Lindsley WG. In: Ashley K,
O’Connor PF, eds. NIOSH Manual of Analytical Methods, 5th edition. Cincinnati, OH: U.S.
Department of Health and Human Services, Centers for Disease Control and Prevention,
National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No. 2014-
151, http://www.cdc.gov/niosh/nmam.
NIOSH [2016c]. General considerations for sampling airborne contaminants. By McCammon
CS Jr., Woebkenberg ML. In: Ashley K, O’Connor PF, eds. NIOSH Manual of Analytical
Methods, 5th edition. Cincinnati, OH: U.S. Department of Health and Human Services,
Centers for Disease Control and Prevention, National Institute for Occupational Safety and
Health, DHHS (NIOSH) Publication No. 2014-151, http://www.cdc.gov/niosh/nmam.
Noris F, Siegel JA, Kinney KA [2011]. Evaluation of HVAC filters as a sampling mechanism
for indoor microbial communities. Atmos Environ 45(2):338-346.
Noss I, Doekes G, Sander I, Heederik DJ, Thorne PS, Wouters IM [2010]. Passive airborne
dust sampling with the electrostatic dustfall collector: optimization of storage and extraction
procedures for endotoxin and glucan measurement. Ann Occup Hyg 54(6):651-658.
Noss I, Wouters IM, Visser M, Heederik DJ, Thorne PS, Brunekreef B, Doekes G [2008].
Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure
assessment. Appl Environ Microbiol 74(18):5621-5627.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-89 of BA-115
Sampling and Characterization of Bioaerosols
Noti JD, Blachere FM, McMillen CM, Lindsley WG, Kashon ML, Slaughter DR, Beezhold DH
[2013]. High humidity leads to loss of infectious influenza virus from simulated coughs. PLoS
ONE 8(2):e57485.
Olenchock SA [2002]. Airborne endotoxin. In: Hurst CJ, Crawford RL, McInerney MJ,
Knudsen GR, Stetzenbach LD, eds. Manual of environmental microbiology. Washington, DC:
ASM Press.
Olenchock SA, Mull JC, Jones WG [1983]. Endotoxins in cotton: washing effects and size
distribution. Am J Ind Med 4(4):515-521.
Otten JA, Morey PR, Burge HA, Chatigny MA, Feeley JC, Peterson K [1986]. Airborne viable
microorganisms in office environments: sampling protocol and analytical procedures.
Proceedings of the 1986 EPA/APCA Symposium on Measurement of Toxic Air Pollutants,
Raleigh, NC: U.S. Environmental Protection Agency and Air Pollution Control Association.
Pan M, Eiguren-Fernandez A, Hsieh H, Afshar-Mohajer N, Hering SV, Lednicky J, Hugh Fan
Z, Wu CY [2016]. Efficient collection of viable virus aerosol through laminar-flow, water-
based condensational particle growth. J Appl Microbiol 120(3):805-815.
Pardon G, Ladhani L, Sandstrom N, Ettori M, Lobov G, Van Der Wijngaart W [2015].
Aerosol sampling using an electrostatic precipitator integrated with a microfluidic interface.
Sens Actuators B Chem 212:344-352.
Park GW, Lee D, Treffiletti A, Hrsak M, Shugart J, Vinje J [2015]. Evaluation of a new
environmental sampling protocol for detection of human norovirus on inanimate surfaces.
Appl Environ Microbiol 81(17):5987-5992.
Parvaneh S, Elfman L, Ahlf E, Nybom R, Elfman LH, van Hage-Hamsten M [2000]. A new
method for collecting airborne allergens. Allergy 55(12):1148-1154.
Pasanen A-L, Yli-Pietila K, Pasanen P, Kalliokoski P, Tarhanen J [1999]. Ergosterol content in
various fungal species and biocontaminated building materials. Appl Environ Microbiol
65(1):138-142.
Pasquarella C, Pitzurra O, Savino A [2000]. The index of microbial air contamination. J Hosp
Infect 46(4):241-256.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-90 of BA-115
Sampling and Characterization of Bioaerosols
Patolsky F, Zheng G, Hayden O, Lakadamyali M, Zhuang X, Lieber CM [2004]. Electrical
detection of single viruses. Proc Natl Acad Sci USA 101(39):14017-14022.
Patolsky F, Zheng G, Lieber CM [2006]. Fabrication of silicon nanowire devices for
ultrasensitive, label-free, real-time detection of biological and chemical species. Nat Protocols
1(4):1711-1724.
Peccia J, Werth HM, Miller S, Hernandez M [2001]. Effects of relative humidity on the
ultraviolet induced inactivation of airborne bacteria. Aerosol Sci Technol 35(3):728-740.
Pityn PJ, Anderson J [2013]. Air sampling of mold spores by slit impactors: yield comparison.
J Environ Sci Health A Tox Hazard Subst Environ Eng 48(12):1485-1490.
Plowman JE [2007]. The proteomics of keratin proteins. J Chromatogr B Analyt Technol
Biomed Life Sci 849(1-2):181-189.
Pöhlker C, Huffman JA, Pöschl U [2012]. Autofluorescence of atmospheric bioaerosols -
Fluorescent biomolecules and potential interferences. Atmos Meas Tech 5(1):37-71.
Poletti L, Pasquarella C, Pitzurra M, Savino A [1999]. Comparative efficiency of nitrocellulose
membranes versus RODAC plates in microbial sampling on surfaces. J Hosp Infect 41(3):195-
201.
Popp W, Zwick H, Rauscher H [1988]. Indirect immunofluorescent test on spore sampling
preparations: a technique for diagnosis of individual mold allergies. Stain Technol 63(4):249-
253.
Portnoy J, Landuyt J, Pacheco F, Flappan S, Simon S, Barnes C [2000]. Comparison of the
Burkard and Allergenco MK-3 volumetric collectors. Ann Allergy Asthma Immunol 84(1):19-
24.
Poulos LM, O'Meara TJ, Hamilton RG, Tovey ER [2002]. Inhaled latex allergen (Hev b 1). J
Allergy Clin Immunol 109(4):701-706.
Poulos LM, O'Meara TJ, Sporik R, Tovey ER [1999]. Detection of inhaled Der p 1. Clin Exp
Allergy 29(9):1232-1238.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-91 of BA-115
Sampling and Characterization of Bioaerosols
Prussin AJ II, Marr LC, Bibby KJ [2014]. Challenges of studying viral aerosol metagenomics
and communities in comparison with bacterial and fungal aerosols. FEMS Microbiol Lett
357(1):1-9.
Pui DYH, Romay-Novas F, Liu BYH [1987]. Experimental study of particle deposition in
bends of circular cross section. Aerosol Sci Technol 7(3):301-315.
Qian J, Hospodsky D, Yamamoto N, Nazaroff WW, Peccia J [2012]. Size-resolved emission
rates of airborne bacteria and fungi in an occupied classroom. Indoor Air 22(4):339-351.
Ravva SV, Hernlem BJ, Sarreal CZ, Mandrell RE [2012]. Bacterial communities in urban
aerosols collected with wetted-wall cyclonic samplers and seasonal fluctuations of live and
culturable airborne bacteria. J Environ Monit 14(2):473-481.
Raynor PC, Leith D, Lee KW, Mukund R [2011]. Sampling and analysis using filters. In:
Kulkarni P, Baron PA, Willeke K, eds. Aerosol measurement: principles, techniques, and
applications. Hoboken, NJ: John Wiley & Sons.
Razmovski V, O'Meara TJ, Taylor DJM, Tovey ER [2000]. A new method for simultaneous
immunodetection and morphologic identification of individual sources of pollen allergens. J
Allergy Clin Immunol 105(4):725-731.
Reed LJ, Muench H [1938]. A simple method of estimating fifty per cent endpoints. Am J Hyg
27:493-497.
Reid TM, Schafer MP [1999]. Direct detection of Histoplasma capsulatum in soil suspensions
by two-stage PCR. Mol Cell Probes 13(4):269-273.
Renstrom A [2002]. Exposure to airborne allergens: a review of sampling methods. J Environ
Monit 4:619-622.
Renstrom A, Karlsson AS, Tovey E [2002]. Nasal air sampling used for the assessment of
occupational allergen exposure and the efficacy of respiratory protection. Clin Exp Allergy
32(12):1769-1775.
Reponen T, Lockey J, Bernstein DI, Vesper SJ, Levin L, Khurana Hershey GK, Zheng S, Ryan
P, Grinshpun SA, Villareal M, Lemasters G [2012]. Infant origins of childhood asthma
associated with specific molds. J Allergy Clin Immunol 130(3):639-644.e5.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-92 of BA-115
Sampling and Characterization of Bioaerosols
Reponen T, Seo S-C, Grimsley F, Lee T, Crawford C, Grinshpun SA [2007]. Fungal fragments
in moldy houses: a field study in homes in New Orleans and Southern Ohio. Atmos Environ
41(37):8140-8149.
Reponen T, Vesper S, Levin L, Johansson E, Ryan P, Burkle J, Grinshpun SA, Zheng S,
Bernstein DI, Lockey J, Villareal M, Khurana Hershey GK, LeMasters G [2011a]. High
environmental relative moldiness index during infancy as a predictor of asthma at 7 years of
age. Ann Allergy Asthma Immunol 107(2):120-126.
Reponen T, Willeke K, Grinshpun S, Nevalainen A [2011b]. Biological particle sampling. In:
Kulkarni P, Baron PA, Willeke K, eds. Aerosol measurement: principles, techniques, and
applications. Hoboken, NJ: John Wiley & Sons.
Reponen T, Willeke K, Ulevicius V, Reponen A, Grinshpun SA [1996]. Effect of relative
humidity on the aerodynamic diameter and respiratory deposition of fungal spores. Atmos
Environ 30(23):3967-3974.
Reponen TA, Gazenko SV, Grinshpun SA, Willeke K, Cole EC [1998]. Characteristics of
airborne actinomycete spores. Appl Environ Microbiol 64(10):3807-3812.
Rintala H, Pitkaranta M, Taubel M [2012]. Microbial communities associated with house dust.
Adv Appl Microbiol 78:75-120.
Rittenour WR, Ciaccio CE, Barnes CS, Kashon ML, Lemons AR, Beezhold DH, Green BJ
[2014]. Internal transcribed spacer rRNA gene sequencing analysis of fungal diversity in
Kansas City indoor environments. Environ Sci Process Impacts 16(1):33-43.
Rittenour WR, Hamilton RG, Beezhold DH, Green BJ [2012]. Immunologic,
spectrophotometric and nucleic acid based methods for the detection and quantification of
airborne pollen. J Immunol Methods 383(1-2):47-53.
Rodes CE, Thornburg JW [2005]. Breathing zone exposure assessment. In: Ruzer LS, Harley
NH, Ruzer LS, Harley NH, eds. Aerosols handbook: measurement, dosimetry, and health
effects. Boca Raton, FL: CRC Press.
Roux J-M, Kaspari O, Heinrich R, Hanschmann N, Grunow R [2013]. Investigation of a new
electrostatic sampler for concentrating biological and non-biological aerosol particles. Aerosol
Sci Technol 47(5):463-471.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-93 of BA-115
Sampling and Characterization of Bioaerosols
Rule AM, Chapin AR, McCarthy SA, Gibson KE, Schwab KJ, Buckley TJ [2005]. Assessment
of an aerosol treatment to improve air quality in a swine concentrated animal feeding
operation (CAFO). Environ Sci Technol 39(24):9649-9655.
Rule AM, Kesavan J, Schwab KJ, Buckley TJ [2007]. Application of flow cytometry for the
assessment of preservation and recovery efficiency of bioaerosol samplers spiked with Pantoea
agglomerans. Environ Sci Technol 41(7):2467-2472.
Ryan E, Wright C, Gloster J [2009]. Measurement of airborne foot-and-mouth disease virus:
preliminary evaluation of two portable air sampling devices. Vet J 179(3):458-461.
Rydjord B, Namork E, Nygaard UC, Wiker HG, Hetland G [2007]. Quantification and
characterisation of IgG binding to mould spores by flow cytometry and scanning electron
microscopy. J Immunol Methods 323:123-131.
Rylander R, Vesterlund J [1982]. Airborne endotoxins in various occupational environments.
Prog Clin Biol Res 93:399-409.
Saari S, Reponen T, Keskinen J [2014]. Performance of two fluorescence-based real-time
bioaerosol detectors: BioScout vs. UVAPS. Aerosol Sci Technol 48(4):371-378.
Salvin SB [1949]. Cysteine and related compounds in the growth of the yeast like phase of
Histoplasma capsulatum. J Infect Dis 84(3):275-283.
Santarpia JL, Ratnesar-Shumate S, Gilberry JU, Quizon JJ [2013]. Relationship between
biologically fluorescent aerosol and local meteorological conditions. Aerosol Sci Technol
47(6):655-661.
Sasser M [1990a]. Identification of bacteria by gas chromatography of cellular fatty acids
(Technical Note #101). Newark, DE: Microbial Identification, Inc.
Sasser M [1990b]. Identification of bacteria through fatty acid analysis. In: Klement Z,
Rudolph K, Sands DC, eds. Methods in phytobacteriology. Budapest, Hungary: Akadémiai
Kiadó.
Schafer MP, Fernback JE, Ernst MK [1999]. Detection and characterization of airborne
mycobacterium tuberculosis H37Ra particles, a surrogate for airborne pathogenic M.
tuberculosis. Aerosol Sci Technol 30(2):161-173.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-94 of BA-115
Sampling and Characterization of Bioaerosols
Schafer MP, Fernback JE, Jensen PA [1998]. Sampling and analytical method development for
qualitative assessment of airborne mycobacterial species of the Mycobacterium tuberculosis
complex. Am Ind Hyg Assoc J 59(8):540-546.
Schafer MP, Martinez KF, Mathews ES [2003]. Rapid detection and determination of the
aerodynamic size range of airborne mycobacteria associated with whirlpools. Appl Occup
Environ Hyg 18(1):41-50.
Schaffer FL, Soergel ME, Straube DC [1976]. Survival of airborne influenza virus: effects of
propagating host, relative humidity, and composition of spray fluids. Arch Virol 51(4):263-
273.
Schnurer J [1993]. Comparison of methods for estimating the biomass of three food-borne
fungi with different growth patterns. Appl Environ Microbiol 59(2):552-555.
Scott JA, Summerbell RC, Green BJ [2011]. Detection of indoor fungi bioaerosols. In: Adnan
O, Samson RA, eds. Fundamentals of mold growth in indoor environments and strategies for
healthy living. Amsterdam, Netherlands: Wageningen Academic Publishers.
Seitz LM, Sauer DB, Burroughs R, Mohr HE, Hubbard JD [1979]. Ergosterol as a measure of
fungal growth. Phytopathology 69:1202-1203.
Seo S, Choung JT, Chen BT, Lindsley WG, Kim KY [2014]. The level of submicron fungal
fragments in homes with asthmatic children. Environ Res 131:71-76.
Shelton BG, Kirkland KH, Flanders WD, Morris GK [2002]. Profiles of airborne fungi in
buildings and outdoor environments in the United States. Appl Environ Microbiol
68(4):1743-1753.
Sigma-Aldrich [1998]. Bulletin 910 Guide to solid phase extraction.
http://www.sigmaaldrich.com/Graphics/Supelco/objects/4600/4538.pdf.
Simon X, Bau S, Boivin A, Duquenne P, Witschger O, Görner P [2016]. Physical
performances and kinetics of evaporation of the CIP 10-M personal sampler's rotating cup
containing aqueous or viscous collection fluid. Aerosol Sci Technol 50(5):507-520.
Singh U, Levin L, Grinshpun SA, Schaffer C, Adhikari A, Reponen T [2011a]. Influence of
home characteristics on airborne and dustborne endotoxin and beta-D-glucan. J Environ
Monit 13(11):3246-3253.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-95 of BA-115
Sampling and Characterization of Bioaerosols
Singh U, Reponen T, Cho KJ, Grinshpun SA, Adhikari A, Levin L, Indugula R, Green BJ
[2011b]. Airborne endotoxin and beta-D-glucan in PM1 in agricultural and home
environments. Aerosol Air Qual Res 11(4):376-386.
Smid T, Schokkin E, Boleij JS, Heederik D [1989]. Enumeration of viable fungi in
occupational environments: a comparison of samplers and media. Am Ind Hyg Assoc J
50(5):235-239.
Solomon WR, Burge HA, Boise JR [1980]. Exclusion of particulate allergens by window air
conditioners. J Allergy Clin Immunol 65(4):305-308.
Soo JC, Monaghan K, Lee T, Kashon M, Harper M [2016]. Air sampling filtration media:
collection efficiency for respirable size-selective sampling. Aerosol Sci Technol 50(1):76-87.
Sorensen CM, Gebhart J, O'Hern TJ, Rader DJ [2011]. Optical measurement techniques:
fundamentals and applications. In: Kulkarni P, Baron PA, Willeke K, eds. Aerosol
measurement: principles, techniques, and applications. Hoboken, NJ: John Wiley & Sons.
Staton SJR, Castillo JA, Taylor TJ, Herckes P, Hayes MA [2013]. Identifying indoor
environmental patterns from bioaerosol material using HPLC. Anal Bioanal Chem
405(1):351-357.
Stewart GL, Parkman PD, Hopps HE, Douglas RD, Hamilton JP, Meyer HM, Jr. [1967].
Rubella-virus hemagglutination-inhibition test. N Engl J Med 276(10):554-557.
Summerbell RC, Green BJ, Corr D, Scott JA [2011]. Molecular methods for bioaerosol
characterization. In: Flannigan B, Samson RA, Miller JD, eds. Microorganisms in home and
indoor work environments: diversity, health impacts, investigation and control, 2
nd
Edition.
Boca Raton, FL: CRC Press, Taylor and Francis Group.
Takahashi Y, Nagoya T, Watanabe M, Inouye S, Sakaguchi M, Katagiri SA [1993]. A new
method of counting airborne Japanese cedar (Cryptomeria japonica) pollen allergens by
immunoblotting. Allergy 48:94-98.
Takahashi Y, Nilsson S [1995]. Aeroallergen immunoblotting with human IgE antibody.
Grana 34:357-360.
Tang JW [2009]. The effect of environmental parameters on the survival of airborne infectious
agents. J R Soc Interface 6(Suppl 6):S737-S746.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-96 of BA-115
Sampling and Characterization of Bioaerosols
Tang W, Kuehn TH, Simcik MF [2015]. Effects of temperature, humidity and air flow on
fungal growth rate on loaded ventilation filters. J Occup Environ Hyg 12(8):525-537.
Taubel M, Karvonen AM, Reponen T, Hyvarinen A, Vesper S, Pekkanen J [2016]. Application
of the environmental relative moldiness index in Finland. Appl Environ Microbiol 82(2):578-
584.
Thermo Fisher Scientific [2014]. Molecular probes handbook—A guide to fluorescent probes
and labeling technologies, 11th Edition. Waltham, MA: ThermoFisher Scientific.
https://www.thermofisher.com/us/en/home/references/molecular-probes-the-
handbook.html.
Thompson KA, Pappachan JV, Bennett AM, Mittal H, Macken S, Dove BK, Nguyen-Van-
Tam JS, Copley VR, O'Brien S, Hoffman P, Parks S, Bentley A, Isalska B, Thomson G,
Consortium ES [2013]. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic-
-the risk of aerosol generation during medical procedures. PLoS ONE 8(2):e56278.
Thorne PS, DeKoster JA, Subramanian P [1996]. Environmental assessment of aerosols,
bioaerosols, and airborne endotoxins in a machining plant. Am Ind Hyg Assoc J 57(12):1163-
1167.
Thorne PS, Metwali N, Avol E, McConnell RS [2005]. Surface sampling for endotoxin
assessment using electrostatic wiping cloths. Ann Occup Hyg 49(5):401-406.
Toivola M, Alm S, Reponen T, Kolari S, Nevalainen A [2002]. Personal exposures and
microenvironmental concentrations of particles and bioaerosols. J Environ Monit 4(1):166-
174.
Toprak E, Schnaiter M [2013]. Fluorescent biological aerosol particles measured with the
Waveband Integrated Bioaerosol Sensor WIBS-4: laboratory tests combined with a one year
field study. Atmos Chem Phys 13(1):225-243.
Tortora GJ, Funke BR, Case CL [2013]. Microbiology - an introduction. Glenview, IL: Pearson
Education.
Tovey E, Taylor D, Graham A, O'Meara T, Lovborg U, Jones A, Sporik R [2000]. New
immunodiagnostic system. Aerobiologia 16(1):113-118.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-97 of BA-115
Sampling and Characterization of Bioaerosols
Tovey ER, Green BJ [2004]. Measuring environmental fungal exposure. Med Mycol 43(suppl
1):S67-S70.
Tovey ER, Liu-Brennan D, Garden FL, Oliver BG, Perzanowski MS, Marks GB [2016]. Time-
based measurement of personal mite allergen bioaerosol exposure over 24 hour periods. PLoS
ONE 11(5):e0153414.
Trouwborst T, Kuyper S [1974]. Inactivation of bacteriophage T3 in aerosols: effect of
prehumidification on survival after spraying from solutions of salt, peptone, and saliva. Appl
Microbiol 27(5):834-837.
Trunov M, Trakumas S, Willeke K, Grinshpun SA, Reponen T [2001]. Collection of
bioaerosol particles by impaction: effect of fungal spore agglomeration and bounce. Aerosol
Sci Technol 35(1):617-624.
Tsai CJ, Pui DYH [1990]. Numerical study of particle deposition in bends of a circular cross-
section laminar-flow regime. Aerosol Sci Technol 12(4):813-831.
Tsai FC, Macher JM [2005]. Concentrations of airborne culturable bacteria in 100 large US
office buildings from the BASE study. Indoor Air 15(Suppl 9):71-81.
Tseng C-C, Li C-S [2005]. Collection efficiencies of aerosol samplers for virus-containing
aerosols. J Aerosol Sci 36(5-6):593-607.
Tseng CC, Chang LY, Li CS [2010]. Detection of airborne viruses in a pediatrics department
measured using real-time qPCR coupled to an air-sampling filter method. J Environ Health
73(4):22-28.
Turgeon N, Toulouse MJ, Martel B, Moineau S, Duchaine C [2014]. Comparison of five
bacteriophages as models for viral aerosol studies. Appl Environ Microbiol 80(14):4242-4250.
Unser S, Bruzas I, He J, Sagle L [2015]. Localized surface plasmon resonance biosensing:
current challenges and approaches. Sensors (Basel) 15(7):15684-15716.
Usachev EV, Usacheva OV, Agranovski IE [2014]. Surface plasmon resonance-based bacterial
aerosol detection. J Appl Microbiol 117(6):1655-1662.
USP [1997]. Microbiological evaluation of clean rooms and other controlled environments.
Pharmacopeial Forum 23(6):5269-5295.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-98 of BA-115
Sampling and Characterization of Bioaerosols
Verreault D, Gendron L, Rousseau GM, Veillette M, Masse D, Lindsley WG, Moineau S,
Duchaine C [2011]. Detection of airborne lactococcal bacteriophages in cheese manufacturing
plants. Appl Environ Microbiol 77(2):491-497.
Verreault D, Moineau S, Duchaine C [2008]. Methods for sampling of airborne viruses.
Microbiol Mol Biol Rev 72(3):413-444.
Vesper S, Barnes C, Ciaccio CE, Johanns A, Kennedy K, Murphy JS, Nunez-Alvarez A, Sandel
MT, Cox D, Dewalt G, Ashley PJ [2013]. Higher Environmental Relative Moldiness Index
(ERMI) values measured in homes of asthmatic children in Boston, Kansas City, and San
Diego. J Asthma 50(2):155-161.
Vesper S, McKinstry C, Haugland R, Wymer L, Bradham K, Ashley P, Cox D, Dewalt G,
Friedman W [2007]. Development of an Environmental Relative Moldiness Index for US
homes. J Occup Environ Med 49(8):829-833.
Vincent JH [2005]. Health-related aerosol measurement: a review of existing sampling criteria
and proposals for new ones. J Environ Monit 7(11):1037-1053.
Vincent JH [2007]. Aerosol sampling: science, standards, instrumentation and applications.
Hoboken, NJ: John Wiley & Sons.
Wagner J, Macher JM [2003]. Comparison of a passive aerosol sampler to size-selective pump
samplers in indoor environments. Am Ind Hyg Assoc J 64(5):630-639.
Walls HJ, Ensor DS, Harvey LA, Kim JH, Chartier RT, Hering SV, Spielman SR, Lewis GS
[2016]. Generation and sampling of nanoscale infectious viral aerosols. Aerosol Sci Technol
50(8):802-811.
Walter MV, Marthi B, Fieland VP, Ganio LM [1990]. Effect of aerosolization on subsequent
bacterial survival. Appl Environ Microbiol 56(11):3468-3472.
Wang H, Reponen T, Lee SA, White E, Grinshpun SA [2007]. Size distribution of airborne
mist and endotoxin-containing particles in metalworking fluid environments. J Occup
Environ Hyg 4(3):157-165.
Wang Z, Reponen T, Grinshpun SA, Gorny RL, Willeke K [2001]. Effect of sampling time and
air humidity on the bioefficiency of filter samplers for bioaerosol collection. J Aerosol Sci
32(5):661-674.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-99 of BA-115
Sampling and Characterization of Bioaerosols
Warren JC, Akers TG, Dubovi EJ [1969]. Effect of prehumidification on sampling of selected
airborne viruses. Appl Microbiol 18(5):893-896.
Weber TP, Stilianakis NI [2008]. Inactivation of influenza A viruses in the environment and
modes of transmission: a critical review. J Infect 57(5):361-373.
West JS, Atkins SD, Emberlin J, Fitt BD [2008]. PCR to predict risk of airborne disease.
Trends Microbiol 16(8):380-387.
Whitehead T, Leith D [2008]. Passive aerosol sampler for particle concentrations and size
distributions. J Environ Monit 10(3):331-335.
WHO [2009]. WHO guidelines for indoor air quality: dampness and mould. Geneva: World
Health Organization.
Wilken JA, Marquez P, Terashita D, McNary J, Windham G, Materna B [2014].
Coccidioidomycosis among cast and crew members at an outdoor television filming event--
California, 2012. MMWR Morb Mortal Wkly Rep 63(15):321-324.
Wilken JA, Sondermeyer G, Shusterman D, McNary J, Vugia DJ, McDowell A, Borenstein P,
Gilliss D, Ancock B, Prudhomme J, Gold D, Windham GC, Lee L, Materna BL [2015].
Coccidioidomycosis among workers constructing solar power farms, California, USA, 2011-
2014. Emerg Infect Dis 21(11):1997-2005.
Willeke K, Lin X, Grinshpun SA [1998]. Improved aerosol collection by combined impaction
and centrifugal motion. Aerosol Sci Technol 28(5):439-456.
Willeke K, Macher JM [1999]. Air sampling. In: Macher JM, ed. Bioaerosols: assessment and
control. Cincinnati, OH: American Conference of Governmental Industrial Hygienists.
Wurtz H, Sigsgaard T, Valbjorn O, Doekes G, Meyer HW [2005]. The dustfall collector--a
simple passive tool for long-term collection of airborne dust: a project under the Danish
Mould in Buildings program (DAMIB). Indoor Air 15(Suppl 9):33-40.
Yang W, Elankumaran S, Marr LC [2011]. Concentrations and size distributions of airborne
influenza A viruses measured indoors at a health centre, a day-care centre and on aeroplanes.
J R Soc Interface 8(61):1176-1184.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-100 of BA-115
Sampling and Characterization of Bioaerosols
Yooseph S, Andrews-Pfannkoch C, Tenney A, McQuaid J, Williamson S, Thiagarajan M,
Brami D, Zeigler-Allen L, Hoffman J, Goll JB, Fadrosh D, Glass J, Adams MD, Friedman R,
Venter JC [2013]. A metagenomic framework for the study of airborne microbial
communities. PLoS ONE 8(12):e81862.
Zhao Y, Aarnink AJ, Wang W, Fabri T, Groot Koerkamp PW, de Jong MC [2014]. Airborne
virus sampling: efficiencies of samplers and their detection limits for infectious bursal disease
virus (IBDV). Ann Agric Environ Med 21(3):464-471.
Zimmerman NJ, Reist PC, Turner AG [1987]. Comparison of two biological aerosol sampling
methods. Appl Environ Microbiol 53(1):99-104.
Zuo ZL, Kuehn TH, Verma H, Kumar S, Goyal SM, Appert J, Raynor PC, Ge S, Pui DYH
[2013]. Association of airborne virus infectivity and survivability with its carrier particle size.
Aerosol Sci Technol 47(4):373-382.

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-101 of BA-115
Sampling and Characterization of Bioaerosols
14 Appendix 1- List of manufacturers/distributors of
common bioaerosol samplers and related
products
This list is not inclusive, and the inclusion of a specific product or company does not
constitute endorsement by the National Institute for Occupational Safety and Health
(NIOSH). If your bioaerosol equipment manufacturing or supply company is not listed or the
information is incorrect or out-of-date, please contact us and we will review your information
for inclusion as the list is updated.
Manufacturers/Distributors of Common Bioaerosol Samplers and Related Products
A.P. Buck Inc.
7101 Presidents Drive, Suite 110
Orlando, FL 32809 USA
Phone: (800) 330-BUCK [2825]
Phone: (407) 851-8602
Fax: (407) 851-8910
http://www.apbuck.com
Ace Glass Incorporated
1430 North West Boulevard
P.O. Box 688
Vineland, NJ 08362 USA
Phone: (800) 223-4524
Phone: (856) 692-3333
Fax: (800) 543-6752
Fax: (856) 692-8919
http://www.aceglass.com
Aerosol Devices Inc.
2614 S. Timberline Road, #109-125
Fort Collins, CO 80525 USA
Phone: (970) 744-3244
http://aerosoldevices.com
Aquaria srl
Via della Levata, 14
Lacchiarella (Milan) 20084 Italy
Phone: +39 02-90091399
Fax: +39 02-9054861
http://www.aquariasrl.com
Barramundi Corporation
6449 South Tex Point
PO Drawer 4259
Homosassa, FL 34448 USA
Phone: (800) 382-1817
Phone: (352) 628-0200
Fax: (352) 628-0203
http://barramundicorp.com
BD Biosciences
2350 Qume Drive
San Jose, CA 95131
Phone: 877-232-8995
http://www.bdbiosciences.com
Beijing SENNON Technology
Development Company, Ltd.
North Building No. 2
Dongdajie xili, Fengtai District
Beijing 100071 P.R. China
Phone: +86 10-6381 8024
Fax: +86 10-6380 6170
http://www.sennon.net/eng
Bertin Corporation
9700 Great Seneca Highway
Suite # 662
Rockville, MD 20850 USA
Phone: (240) 428-1047
http://www.coriolis-airsampler.com
Bi-Air Corporation
1349 Montevideo Ave
Placentia, CA 92870 USA
Phone: (714) 985-9659
Fax: (714) 528-5429
http://expertonmold.com
bioMérieux, Inc.
595 Anglum Road
Hazelwood, MO 63042 USA
Phone: (800) 634-7656
Fax: (800) 657-3053
http://www.biomerieux-usa.com
Bioscience International
11333 Woodglen Drive
Rockville, MD 20852 USA
Phone: (301) 231-7400
Fax: (301) 231-7277
http://www.biosci-intl.com
Burkard Manufacturing Company
Ltd.
Woodcock Hill Industrial Estate
Rickmansworth, Hertfordshire WD3
1PJ England
Phone: +44 (0) 1923 773134
Fax: +44 (0) 1923 774790
http://www.burkard.co.uk
Climet Instruments Company
1320 W. Colton Avenue
Redlands, CA 92374 USA
Phone: (909) 793-2788
http://www.climet.com
Droplet Measurement Technologies
2545 Central Avenue
Boulder, CO 80301 USA
Phone: (303) 440-5576Fax: (303) 440-
1965
http://www.dropletmeasurement.com

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-102 of BA-115
Sampling and Characterization of Bioaerosols
Manufacturers/Distributors of Common Bioaerosol Samplers and Related Products -
Continued
Dycor Technologies, Ltd.
1851 94th Street
Edmonton, AB T6N 1E6 Canada
Phone: (800) 663-9267
Phone: (780) 486-0091
Fax: (780) 486-3535
http://www.dycor.com
EMD Millipore
290 Concord Road
Billerica, MA 01821 USA
Phone: (800) MILLIPORE [645-5476]
Fax: (781) 533-6000
http://www.emdmillipore.com
EMSL Analytical, Inc
200 Route 130 North
Cinnaminson, NJ 08077
Phone: (800) 220-3675
http://www.emsl.com Environics Oy
Environmental Monitoring Systems, Inc.
3864 Leeds Avenue
Charleston, SC 29405 USA
Phone: (800) 293-3003
Phone: (843) 724-5708
Fax: (866) 724-5702
Fax: (843) 724-5702
http://www.emssales.net
Evogen, Inc.
10513 W. 84th Terrace
Lenexa, KS 66214 USA
Phone: (888) 450-4321
Phone: (913) 948-5640
Fax: (913) 948-5664
http://evogen.com
F.W. Parrett Limited
7 Coppergate Close
Bromley, Kent BR1 3JG England
Phone: +44 020-8460-2116
Fax: +44 020-7504-3536
http://www.parrett.uk.com
FLIR Systems, Inc.
70 Castilian Drive
Goleta, CA 93117 USA
Phone: (888) 747-FLIR [3547]
http://www.flir.com
GE Healthcare Life Sciences
(Whatman)
800 Centennial Avenue
P.O. Box 1327
Piscataway, NJ 08855 USA
Phone: (800) 526-3593
Fax: (877) 295-8102
http://www.gelifesciences.com
Indoor Biotechnologies Inc
700 Harris Street
Charlottesville, VA 22903 USA
https://inbio.com
InnovaPrep
132 East Main Street
Drexel, MO 65742 USA
Phone: (816) 619-3375
http://innovaprep.com
InnovaTek, Inc.
3100 G. Washington Way
Suite 108
Richland, WA 99354 USA
Phone: (509) 375-1093
Fax: (509) 375-5183
http://www.innovatek.com
Inspirotec
2319 West Wabansia Avenue #1
Chicago, IL 60647
Phone: 847 302 1839
Fax: 847 234 2089
http://www. inspirotec.com
MSP Corporation
5910 Rice Creek Parkway
Suite 300
Shoreview, MN 55126 USA
Phone: (651) 287-8100
Fax: (651) 287-8140
http://www.mspcorp.com
Pall Corporation
25 Harbor Park Drive
Port Washington, NY 11050 USA
Phone: (800) 521-1520
Phone: (516) 484-3600
Fax: (516) 801-9754
http://www.pall.com
Particle Measuring Systems
5475 Airport Boulevard
Boulder, CO 80301 USA
Phone: (800) 238-1801
Phone: (303) 443-7100
Fax: (303) 449-6870
http://pmeasuring.com
Research International, Inc.
17161 Beaton Road SE
Monroe, WA 98272 USA
Phone: (800) 927-7831
Phone: (360) 805-4930
Fax: (360) 863-0439
http://www.resrchintl.com
RJ Lee Group, Inc.
350 Hochberg Road
Monroeville, PA 15146
Manufacturers/Distributors of
Common Bioaerosol Samplers and
Related Products
724) 325-1776
Fax: (724) 733-1799
http://www.rjlg.com
Sammonkatu 12
P.O. Box 349
FI-50101 Mikkeli Finland
Phone: +358 201 430 430
Fax: +358 201430 440
http://www.environics.fi/
Sartorius Stedim Biotech GmbH
August-Spindler-Strasse 11
Goettingen 37079 Germany
Phone: (800) 368-7178
Phone: +49 551-308-0
Fax: +49 551-308-3289
https://www.sartorius.com
SKC, Inc.
863 Valley View Road
Eighty Four, PA 15330 USA
Phone: (800) 752-8472
Phone: (724) 941-9701
Fax: (724) 941-1369
http://www.skcinc.com

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-103 of BA-115
Sampling and Characterization of Bioaerosols
Manufacturers/Distributors of Common Bioaerosol Samplers and Related Products -
Continued
Tecora
211-215 Rue la Fontaine
94134 Fontenay sous Bois Cedex, France
Phone: Tel: +33 1 48 75 82 82
Fax: +33 1 48 75 82 96
http://www.tecora.com/en/
Thermo Scientific
27 Forge Parkway
Franklin, MA 02038 USA
Phone: (866) 282-0430
Phone: (508) 520-0430
Fax: (508) 520-1460
http://www.thermoscientific.com
TSI Incorporated
500 Cardigan Road
Shoreview, MN 55126 USA
Phone: (800) 874-2811
Phone: (651) 483-0900
Fax: (651) 490-3824
http://www.tsi.com
Veltek Associates, Inc.
15 Lee Boulevard
Malvern, PA 19355 USA
Phone: (888) 4-STERILE [478-3745]
Phone: (610) 644-8335
http://sterile.com
Zefon International, Inc.
5350 SW 1st Lane
Ocala, FL 34474 USA
Phone: (800) 282-0073
Phone: (352) 854-8080
Fax: (352) 854-7480
http://www.zefon.com
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-104 of BA-115
Sampling and Characterization of Bioaerosols
15 Appendix 2 – Commonly used bioaerosol samplers
This list is not inclusive, and the inclusion of a specific product or company does not constitute endorsement by the National Institute for
Occupational Safety and Health (NIOSH). If a bioaerosol sampler is not listed or the information is incorrect or out-of-date, please contact
us and we will review your information for inclusion as the list is updated.
d
50
– aerodynamic diameter at which the collection efficiency is 50% and is defined as the cut off diameter
Application
¥
-
C = culture-based analysis for viability
M = microscopic examination of collected bioaerosol
O = other laboratory analyses, such as immunoassays, bioassays, chemical assays, or molecular detection techniques
Table I. Common Commercially-Available Filter Samplers for Bioaerosol Collection
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
d
50
(µm)
Application
¥
Bi-Air
Bi-Air Filter Cassette
Filter
1 – 5
Varies
C,M,O
Burkard
High Throughput 'Jet' Spore and Particle Sampler
Filter
850
Varies
C,M,O
Dycor
CSU-1 Low Volume Air Sampler
Filter
10
Varies
C,M,O
EMD Millipore
Various filter and membrane media
Filter
Varies
Varies
C,M,O
GE (Whatman)
Various filter and membrane media
Filter
Varies
Varies
C,M,O
InnovaPrep
ACD-200 Bobcat Air Sampler
Filter
100 or 200
C,M,O
Pall Corp.
Various filter and membrane media
Filter
Varies
Varies
C,M,O
Research
International
SASS® 3100 Dry Air Sampler Filter 50 – 310 0.3 – 0.5 C,M,O
Sartorius
AirPort MD8 Air Sampler
Filter
30, 40, or 50
-
C
SKC
Button Aerosol Sampler
Filter
4
-
M,O
Zefon
Various filter and membrane media
Various
Varies
Varies
C,M,O
Table II A. Common Commercially-Available Single-stage Impactor Samplers for Bioaerosol Collection
Manufacturer/
Collection
Flowrate
d
50
Sampler Name Application
¥
Distributor
Media
(L/min)
(µm)
BioAire™ B6 Single Stage Microbial Sampler
Agar
28.3
-
C
A.P. Buck
Bio-Culture™ Microbial Air Sampler
Agar
30 – 120
-
C
BioSlide™ Microbial Air Sampler
Slide
10 – 20
-
M
MICROFLOW 60 Microbiological Air Sampler
Agar
30 – 120
-
C
Aquaria
MICROFLOW 60-90/C Microbiological Air Sampler
Agar
30 – 120
-
C
MICROFLOW 90/C Microbiological Air Sampler
Agar
30 – 120
-
C
Barramundi
Mattson-Garvin Model 220 Air Sampler (240V is Model 270)
Agar
28.3
-
C
Handy Microbial Air Sampler®
Agar/Filter
-
-
C,M,O
Beijing
JWL-IIA Mini® Microbial Air Sampler
Agar
20
-
C
SENNON
JWL-IIB202 Universal® Microbial Air Sampler
Agar
20
-
C
JWL-IIC Professional® Microbial Air Sampler
Agar
20
-
C
airIDEAL® 3P™ Traceability Air Sampler
Agar
100
-
C
bioMérieux
Samp'air™ Microbial Air Sampler
Agar
100
-
C
SAS Duo 360 High Volume Microbial Air Sampler
Agar
360
-
C
Bioscience
SAS Isolator Microbial Air Sampler
Agar
180
-
C
International
SAS Super 100 Microbial Air Sampler
Agar
100
-
C
SAS Super 180 Microbial Air Sampler
Agar
180
-
C
24-Hour Recording Volumetric Spore Trap
Slide
10
-
M
Continuous Recording Air Sampler
Slide
10
-
M
Personal Volumetric Air Sampler
Slide
10
-
M
Burkard
Portable Air Sampler for Agar Plates
Agar
10 or 20
-
C
Recording Air Sampler
Side
10
-
M
Seven-Day Recording Volumetric Spore Trap
Slide
10
-
M
CI-90 & CI-90+ Airborne Microbial Sampler
Agar
100
-
C
Climet
CI-95 & CI-95+ Airborne Microbial Sampler
Agar
100
-
C
CI-99 Microbial Air Sampler
Agar
100
-
C
Dycor
Dycor Slit Sampler
Agar
15 – 50
-
C
MAS-100 Iso MH® Air Sampler
Agar
100
-
C
MAS-100 Iso NT® Air Sampler
Agar
100
-
C
MAS-100 NT® and MAS-100 NT Ex® Air Sampler
Agar
100
-
C
EMD Millipore
MAS-100 VF® Active Air Sampler
Agar
100
-
C
RCS® Isolator Microbial Air Sampler
Agar
100
-
C
RCS® Plus Ex Explosion-Proof Microbial Air Sampler
Agar
100
-
C
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-105 of BA-115
Sampling and Characterization of Bioaerosols
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-106 of BA-115
Sampling and Characterization of Bioaerosols
Table II A. Common Commercially-Available Single-stage Impactor Samplers for Bioaerosol Collection - Continued
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
d
50
(µm)
Application
¥
EMD Millipore
RCS® High Flow Touch Portable Air Sampler
Agar
100
-
C
Environmental
Monitoring
Systems
Allergenco MK-III Slit-Impaction Sampler
Slide
15
-
M
Allergenco-D Disposable IAQ Air Monitoring Cassettes
Slide
15
-
M
Allergenco-D Posi-Track Full Slide, IAQ Impactor Cassette
Slide
15
1.7
M
BioSIS Slit Impaction Air Sampler
Slide
5 – 50
-
M
cyclex-d Cassettes
Slide
20
< 1.0
M
E6 Single-Stage Bioaerosol Impaction Sampler
Agar
28.3
-
C
Micro5 MicroCell Cassettes
Slide
5
< 1.0
M
Micro5 Posi-Track Full Slide, IAQ Impactor Cassette
Slide
5
<1.0
M
F.W. Parrett
MicroBio MB1 Air Sampler
Agar
100
-
C
MicroBio MB2 Air Sampler
Agar
100
-
C
Particle
Measuring
Systems
Air Trace® Environmental Slit-to-Agar Sampler
Agar
28.3
-
C
BioCapt™ Impactor Active Microbial Air Sampling Atrium
Agar
25
-
C
MiniCapt™ Portable Microbial Air Sampler
Agar
50 or 100
-
C
Sartorius
AirPort MD8 Air Sampler
Agar
125
-
C
SKC
BioStage® Standard Single-Stage Viable Cascade Impactor
Agar
28.3
-
C
BioStage® 200 Single-Stage Viable Cascade Impactor
Agar
14.15
-
C
VersaTrap® Spore Trap Cassette
Slide
15
-
M
Thermo Scientific
IUL Basic Air Air Sampler
Agar
60 – 100
-
C
IUL Spin Air Air Sampler
Agar
60 – 100
-
C
IUL Spin Air Basic Air Sampler
Agar
60 – 100
-
C
N6 Single-Stage Viable Andersen Impactor
Agar
28.3
0.65
C
Oxoid Air Sampler
Agar
100
-
C
Veltek
SMA MicroPortable® Air Sampler
Agar
1 or 5
-
C
Zefon
International
A-6 Bioaerosol Impactor
Agar
28.3
0.65
C
Air-O-Cell® Sampling Cassette
Slide
15
2.6
M
Via-Cell® Bioaerosol Sampling Cassette
Slide
15
1.56
C,M,O
d
50
– aerodynamic diameter at which the collection efficiency is 50% and is defined as the cut off diameter
Application
¥
-
C = culture-based analysis for viability
M = microscopic examination of collected bioaerosol
O = other laboratory analyses, such as immunoassays, bioassays, chemical assays, or molecular detection techniques
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-107 of BA-115
Sampling and Characterization of Bioaerosols
Table II B. Common Commercially-Available Multi-stage Impactor Samplers for Bioaerosol Collection
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
0.18
0.32
0.56
Model 100-NR (non-rotating)/100-R
Impactor
(rotating) MOUDI™
Filter 30 8
1.0
1.8
3.2
M,O
5.6
10
0.056
0.10
0.18
0.32
MSP
Corporation
Model 110-NR (non-rotating)/100-R
Impactor
(rotating) MOUDI™
Filter 30 10
0.56
1.0
1.8
M,O
3.2
5.6
10
1.0
Model 100-S4 MOUDI™ Impactor Filter 30 3 2.5
10
M,O
0.010
Model 115 Nano-MOUDI™ Impactor Filter 10 3 0.018
0.032
M,O
0.010
Model 116 Nano-MOUDI™ Impactor Filter 30 3 0.018
0.032
M,O
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-108 of BA-115
Sampling and Characterization of Bioaerosols
Table II B. Common Commercially-Available Multi-stage Impactor Samplers for Bioaerosol Collection - Continued
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
MSP
C
orporation
continued
Model 120 R (rotating) Moudi-II™ Impactor Filter 30 10
0.056
0.10
0.18
0.32
0.56
1.0
1.8
3.2
5.6
10
M,O
Model 122-N
R/122-R Moudi-II™ and NanoMoudi-II™ Impactor Filter 30 13
0.010
0.018
0.032
0.056
0.10
0.18
0.32
0.56
1.0
1.8
3.2
5.6
10
M,O
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-109 of BA-115
Sampling and Characterization of Bioaerosols
Table II B. Common Commercially-Available Multi-stage Impactor Samplers for Bioaerosol Collection - Continued
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
0.010
0.018
0.032
0.056
0.10
0.18
Model 125-NR/125-R Moudi-II™ and NanoMoudi-II™ Impactor Filter 10 13
0.32
0.56
M,O
1.0
1.8
3.2
MSP
Corporation
continued
5.6
10
Model 135-6 MiniMOUDI™ (Marple Personal II) Impactor Filter 2 6
0.56
1.0
1.8
3.2
M,O
5.6
10
0.18
0.32
0.56
Model 135-8 MiniMOUDI™ (Marple Personal II) Impactor Filter 2 8
1.0
1.8
M,O
3.2
5.6
10
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-110 of BA-115
Sampling and Characterization of Bioaerosols
Table II B. Common Commercially-Available Multi-stage Impactor Samplers for Bioaerosol Collection - Continued
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
MSP
C
orporation
continued
Model 135-10 MiniMOUDI™ Impactor Filter 2 10
0.056
0.10
0.18
0.32
0.56
1.0
1.8
3.2
5.6
10
M,O
Model 135-13
MiniMOUDI™ Impactor Filter 2 13
0.010
0.018
0.032
0.056
0.10
0.18
0.32
0.56
1.0
1.8
3.2
5.6
10
M,O
Thermo
S
cientific
Eight Stage Non-Viable Cascade Impactor Filter 28.3 8
0.43
0.65
1.1
2.1
3.3
4.7
5.8
9.0
M,O
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-111 of BA-115
Sampling and Characterization of Bioaerosols
Table II B. Common Commercially-Available Multi-stage Impactor Samplers for Bioaerosol Collection - Continued
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
3.5
Marple 294 Personal Cascade Impactor Filter 2 4
9.8
14.8
M,O
21.3
0.52
0.93
Marple 296 Personal Cascade Impactor Filter 2 6
1.55
3.5
M,O
6.0
9.8
0.52
Thermo
Scientific
continued
Marple 298 Personal Cascade Impactor Filter 2 8
0.93
1.55
3.5
6.0
M,O
9.8
14.8
21.3
0.65
1.1
Six Stage Viable Andersen Cascade Impactor Agar 28.3 6
2.1
3.3
C
4.7
7.0
Two-Stage Viable Andersen Cascade Impactor Agar 28.3 2
0.8
8
C
d50 – aerodynamic diameter at which the collection efficiency is 50% and is defined as the cut off diameter
Application¥ -
C = culture-based analysis for viability
M = microscopic examination of collected bioaerosol
O = other laboratory analyses, such as immunoassays, bioassays, chemical assays, or molecular detection techniques
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-112 of BA-115
Sampling and Characterization of Bioaerosols
Table III. Common Commercially-Available Cyclones and Impinger Samplers for Bioaerosol Collection
Cyclones
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
Burkard
Cyclone Sampler for Airborne Particles
Dry Vial
20
1
-
C,M,O
Cyclone Sampler for Field Operation
Dry Vial
16.5
1
-
C,M,O
Evogen
Sceptor DryClone™
Dry Vial
400
1
-
C,M,O
FLIR Systems
C100 Modular Tactical Collector
Dry Vial
150
1
-
C,M,O
Impingers
Ace Glass
AGI-30 Impinger
Liquid
12.5
1
-
C,M,O
Greenburg-Smith Impinger
Liquid
28.3
1
-
C,M,O
Midget Impinger
Liquid
1
-
C,M,O
4
Burkard Multistage Liquid Impinger Liquid 20 3 10
> 10
C,M,O
Dycor
XMX/102 High Volume Bioaerosol Sampling System
Serum Tube
530
1
-
C,O
XMX/2L-MIL Bioaerosol Sampler – Military
Liquid
530
1
-
C,M,O
XMX-CV Microbial Air Sampler – Civilian
Liquid
530
1
-
C,M,O
d
50
– aerodynamic diameter at which the collection efficiency is 50% and is defined as the cut off diameter
Application
¥
-
C = culture-based analysis for viability
M = microscopic examination of collected bioaerosol
O = other laboratory analyses, such as immunoassays, bioassays, chemical assays, or molecular detection techniques
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-113 of BA-115
Sampling and Characterization of Bioaerosols
Table IV. Common Commercially-Available Wetted-Surface and Condensation-Based Samplers for Bioaerosol Collection
Wetted-Surface Bioaerosol Samplers
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
Bertin
Coriolis® µ Microbial Air Sampler
Liquid
100 – 300
1
< 0.5
C,M,O
Coriolis® RECON Portable Air Sampler
Liquid
600
1
≈ 0.5
C,M,O
Bioscience
International
SAS Cyclone Air Sampler Liquid 1200 1 - C,M,O
Evogen
Sceptor SpinCon™ Advanced Air Sampler
Liquid
450
1
-
C,M,O
FLIR Systems Fido® B1 (BioCapture® 650) Portable Air Sampler
Liquid
Cartridge
200 1 - C,M,O
InnovaPrep
SpinCon® II Advanced Air Sampler
Liquid
450
1
-
C,M,O
InnovaTek BioGuardian® Air Sampler Liquid
100, 350,
or 1000
1 - C,M,O
Research
International
BioHawk® 8-Channel Collector/Bioidentifier
Liquid
325
1
-
O
SASS® 2300 Wetted-Wall Air Sampler
Liquid
325
1
-
C,M,O
SASS® 2400 Low-Volume Wetted-Wall Air Sampler
Liquid
40
1
-
C,M,O
SKC
BioSampler®
Liquid
12.5
1
-
C,M,O
Tecora
CIP10-M personal bioaerosol sampler
Liquid
10
1
2.1
C,M,O
Condensation-Based Bioaerosol Samplers
Aerosol Devices
LSS100 Series Liquid Spot Sampler
Liquid
1.0 – 1.5
1
n/a
C,M,O
d
50
– aerodynamic diameter at which the collection efficiency is 50% and is defined as the cut off diameter
Application
¥
-
C = culture-based analysis for viability
M = microscopic examination of collected bioaerosol
O = other laboratory analyses, such as immunoassays, bioassays, chemical assays, or molecular detection techniques
NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-114 of BA-115
Sampling and Characterization of Bioaerosols
Table V. Common Commercially-Available Electrostatic, Passive Aerosol and Settled Dust Collection Samplers for Bioaerosol Collection
Electrostatic Samplers
Manufacturer/
Distributor
Sampler Name
Collection
Media
Flowrate
(L/min)
# of
Stages
d
50
(µm)
Application
¥
Inspirotec
Inspirotec sampler
Cartridge
130
1
n/a
O
Passive electrostatic dust collectors are listed under Passive Aerosol Samplers. Electrostatic cloths used for
Settled Dust Collection Devices.
wipe sampling are listed under
Passive Aerosol Samplers
BD Biosciences
Ready-to-use settle plates
Agar
n/a
n/a
n/a
C
EMD Millipore
Ready-to-use settle plates
Agar
n/a
n/a
n/a
C
Thermo-Scientific
Ready-to-use settle plates
Agar
n/a
n/a
n/a
C
Department of Occupational and
Environmental Health, University of Iowa
Electrostatic Dust Collector (EDC)
Electrostatically-
charged cloths
n/a
n/a
n/a
O
RJ Lee Group
UNC Passive Aerosol Sampler
Various
substrates
n/a
n/a
n/a
M, O
Settled Dust Collection Devices
Indoor Biotechnologies
DUSTREAM® Collector (DU-ST-1)
40 µm nylon
mesh filter
n/a
n/a
n/a
O
d50 – aerodynamic diameter at which the collection efficiency is 50% and is defined as the cut off diameter
Application¥ -
C = culture-based analysis for viability
M = microscopic examination of collected bioaerosol
O = other laboratory analyses, such as immunoassays, bioassays, chemical assays, or molecular detection techniques

NIOSH Manual of Analytical Methods 5th Edition Chapter BA March 2017 Page BA-115 of BA-115
Sampling and Characterization of Bioaerosols
Table VI. Common Commercially-Available Real-Time Bioaerosol Monitors for Bioaerosol Collection
Manufacturer/
Distributor
Sampler Name Detection Method
Flowrate
(L/min)
Application
¥
Bioscience
International
SAS-PCR Pathogenic Microorganisms Air Sampler PCR - R
Droplet
Measurement
Technologies
Wideband Integrated Bioaerosol Sensor (WIBS) Fluorescence 0.3 R
Dycor
C-FLAPS Biological Detection System
Fluorescence
350
R
Environics
ENVI BioScout™
Fluorescence
2
R
FLIR Systems
Fido® B2 Instantaneous Biological Aerosol Detector
Fluorescence
3.8
R
Particle
Measuring
Systems
BioLaz™ Real-Time Microbial Monitor Fluorescence 3.6 R
Research
International
BioHawk® 8-Channel Collector/Bioidentifier
Fluorometric Bioassay
325
R
TacBio™ Biological Aerosol Detector
Fluorescence
1
R
TSI
BIOTRAK® Real-Time Viable Particle Counter 9510-BD
Fluorescence
28.3
R
Fluorescence Aerosol Particle Sensor (FLAPS) 3317 (FLAPS III)™
Fluorescence
1
R
Application
¥
-
R= Real-time or near-real time bioaerosol detection
PCR= Polymerase chain reaction
